28.7: Color Photography
- Page ID
- 22383
Subtractive Color Process
Photography is a popular activity for many, but relatively few have an understanding of the chemistry involved, particularly in color photography. This is unfortunate because color photography represents an interesting combination of photochemistry (energy transfer), organic chemistry (dye formation), optics, psychology and physiology (color perception), and engineering (production and development of film).
The sensation of full color in color transparencies produced by photographic means is achieved by subtraction. As a simple example, let us suppose that the subject to be photographed is blue. To obtain a blue image by shining white light through a transparency, the transparency is made to subtract (absorb) yellow light - that is, to absorb strongly in the \(580\)-\(\text{nm}\) region. How a color image of the subject is recorded chemically on the film and how the film is developed into a transparency will become clearer from the following discussion.
Color Film
The emulsion of a typical color film has three silver-bromide layers separately sensitized by suitable dyes to blue, green, and red light (Figure 28-11). When processed (Section 28-6C), the color formed in each layer is complementary to the color to which the layer is sensitive. Thus, if unexposed film is processed, intense yellow, magenta, and cyan colors are respectively formed in the blue-, green-, and red-sensitive layers. Then, when white light strikes this processed film, the yellow layer subtracts the blue, the magenta subtracts the green, and the cyan subtracts the red, with the result that the film appears black (or nearly so), as corresponds to no exposure to light. However, if the film is exposed to strong blue light before processing, the blue-sensitive layer responds, and when the film is processed, no yellow dye is formed in the blue-sensitive layer (see Figure 28-11). The transparency then contains only the subtraction colors, magenta and cyan. When white light enters a transparency of superimposed magenta and cyan dyes, only blue light is transmitted, as befits the color of the original sensitizing light. (From the right side of Figure 28-10, we see the overlap of 5 and 4 leads to blue.) Similarly, exposure of the film to strong yellow light (containing no blue), followed by processing, results in formation of yellow dye and no magenta nor cyan. This is because the green- and red-sensitive emulsions both are sensitive to yellow light, while the blue-sensitive emulsion does not respond to yellow light.
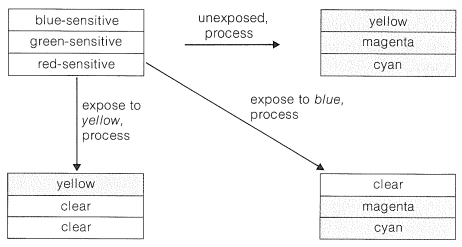
In summary, the overall process from color film to the projection of a color image involves two separate conversions of each color into its complement, the net result being an image that has the same colors as the original subject.
Chemistry of Color Developers
We have seen that full color perception can be achieved by subtraction methods using dyes in suitable combinations. We now have to consider how such dyes are formed on exposure and development of color film. First though, you should recognize that a photographic emulsion, whether for color or black-and-white film, is light-sensitive primarily because of the presence of sliver halide. You will recall from previous discussions (Section 26-2C) that the sequence from exposure to development involves the following:
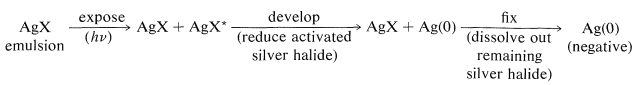
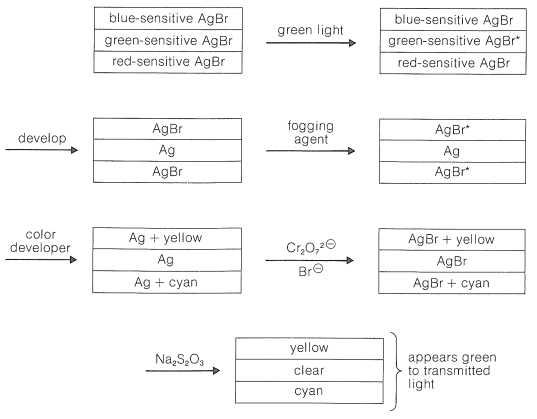
The chemistry involved in the formation of the dyes in the usual color films is highly ingenious and is achieved through steps shown in Figure 28-12 which, for purposes of illustration, are carried through for an initial exposure to green light. The exposure activates only the green-sensitive emulsion, and development with an ordinary developer such as metol-hydroquinone (Section 26-2C) produces silver metal only in the green-sensitive layer. The film now has the visual appearance of a milky negative. The developer then is washed out and the film is fogged, a process that activates the silver bromide remaining unreduced in the first step. The activated silver bromide so formed then is reduced with a color developer, usually 4-amino-\(\ce{N}\),\(\ce{N}\)-diethylbenzenamine, in the presence of a color coupler. Production of dye occurs in direct proportion to the amount of activated silver bromide present, in close conformity with the following equation:
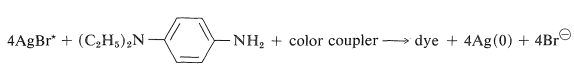 \(\tag{28-13}\)
\(\tag{28-13}\)
A different color coupler is used for each layer of the emulsion and, although the complete color picture is formed in this step, the film is coal black because of the metallic silver produced at the same time. The silver then is oxidized to silver bromide with dichromate solution containing bromide ion and removed with thiosulfate solution. The final image thus contains no silver.
The color-forming reactions obviously are critical to the success of the overall process and, of necessity, involve some degree of compromise between requirements for yield, reproducibility, suitability of color, and light-fastness. In reactions of the type shown in Equation 28-13, the color coupler is a methylene compound, \(\ce{R_2CH_2}\), in which the \(\ce{R}\) groups have sufficient electron-attracting character to undergo some degree of formation of \(\ce{R_2CH}^\ominus\) in the alkaline medium used for color development. The first two steps in the overall sequence follow:
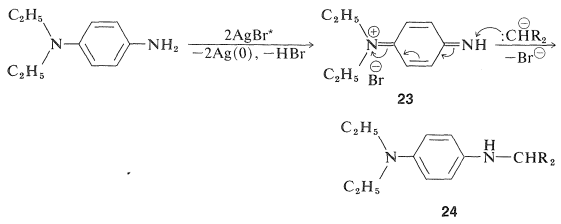
The product \(24\) is a photographic developer and is oxidized either by activated silver bromide or by \(23\) to a new quinonimmonium salt, \(25\):
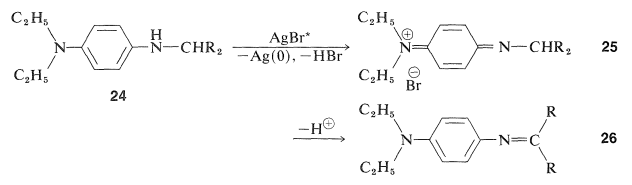
This substance has an acidic hydrogen at the \(\ce{-CHR_2}\) and, on loss of this proton, a neutral conjugated imine results, \(26\), which is the actual dye. Color is expected for these molecules because the \(\ce{R}\) groups are electron-attracting and the diethylamino group at the other end of the conjugated system is electron-donating. The remaining important question is how to adjust the \(\ce{R}\) groups to obtain the proper colors in each layer of the film. Compounds that give yellow, magenta, and cyan dyes with 4-amino-\(\ce{N}\),\(\ce{N}\)-diethylbenzenamine and activated silver bromide are shown below.
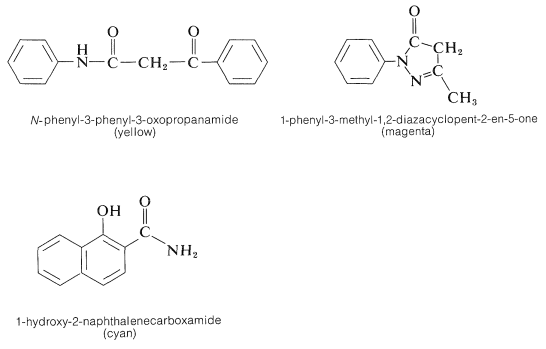
In the case of \(\ce{N}\)-phenyl-3-phenyl-3-oxopropanamide, the yellow dye formed has the following structure:
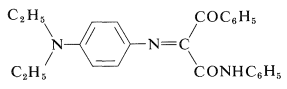
Such dyes often are called azomethines. The nature of the exact color couplers used in color film is not well publicized. It is important that the couplers not diffuse out of the layers where they belong and, for this reason, insolubilizing substituent groups are attached to strategic positions on the color couplers.
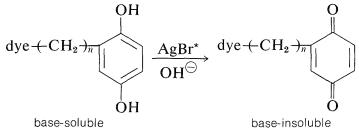
The "instant" color process pioneered by Polaroid operates by a wonderfully ingenious, subtractive-color, three-layer scheme basically similar to the one shown in Figures 28-11 and 28-12. A very important difference is that the colors are transferred from the emulsion to a layer of white pigment \(\left( \ce{TiO_2} \right)\), so the process actually is a printing process. The subtractive dyes (yellow, magenta, and cyan) are present in the emulsion as substituent groups on base-soluble photographic developers, schematically [dye\(\ce{-(CH_2)}_n-\)developer]. A copper phthalocyanine derivative (Section 28-4B) is used to provide the cyan color.
What occurs in a given layer of the film is roughly as follows: Activation of the silver bromide in the layer by the light to which it is sensitized, and then development with an alkaline developer solution converts the "dye developer" to a base-insoluble form (Section 26-2C) in proportion to the light absorbed:
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


