28.4: Chemiluminescence
- Page ID
- 22386
The most common means of generating electronically excited states of molecules is by the absorption of electromagnetic radiation. But excited states are accessible by other routes. Indeed, as shown in Section 28-2E, the excited singlet state of molecular oxygen can be produced by chemical reactions (Equations 28-9 and 28-10). Many other reactions are known that generate products in electronically excited states, and this is especially evident when the electronically excited products go to the ground state by the emission of visible light. This behavior is known as chemiluminescence and is transduction of chemical energy \(\left( \Delta H \right)\) into radiant energy \(\left( h \nu \right)\). Chemiluminescence is possible only when the \(\Delta H\) of the reaction is sufficiently large to allow for production of at least one of the products in an electronically excited state \(\left( ^* \right)\). Chemiluminescence amounts to \(\Delta H \rightarrow ^* \rightarrow h \nu\), which is opposite to most photochemistry which involves \(h \nu \rightarrow ^* \rightarrow \Delta H\).
A beautiful example of a chemiluminescent reaction is the thermal dissociation of the cyclic peroxide, \(8\), into two molecules of 2-propanone:
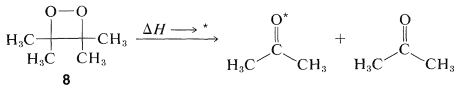 \(\tag{28-11}\)
\(\tag{28-11}\)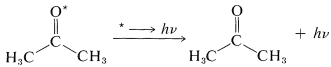
We should not be surprised at the high exothermicity of Reaction 28-11. The peroxide is of high energy (thermochemically unstable) because it combines the strain-energy characteristics of small rings with the weakness of \(\ce{O-O}\) bonds, whereas the product is a stable substance with a strong carbonyl bond.
Chemiluminescence in many reactions is hard to detect because the efficiency of light emission is low. Thus, even though the excited state may be formed in high yield, it may be quenched by other species more efficiently than it loses energy by emission. This fact can be used to advantage by adding a substance that quenches the excited state efficiently and, after energy transfer, gives bright fluorescence or phosphorescence:
\[\Delta H \rightarrow ^* \underset{\text{energy transfer}}{\overset{\text{dye}}{\longrightarrow}} \text{dye}^* \overset{\text{luminescence}}{\longrightarrow} \text{dye} + h \nu\]
Chemiluminescence can be greatly amplified by this process and it forms the basis of spectacular demonstrations of "cold light". An example is the perhydrolysis of ethanedioic (oxalic) esters with hydrogen peroxide in the presence of a fluorescent substance (Equation 28-12). The reaction is believed to pass through the highly unstable dioxacyclobutanedione, which dissociates into two moles of carbon dioxide with such exothermicity that electronic excitation occurs, as evident from the intense light produced in the presence of fluorescent dyes:
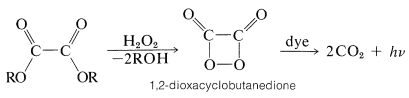 \(\tag{28-12}\)
\(\tag{28-12}\)
This reaction has been developed into a commercial product, marketed under the trade name "Coolite", which can be used as an emergency light source by simply shaking a tube to bring the reactants in contact with one another.
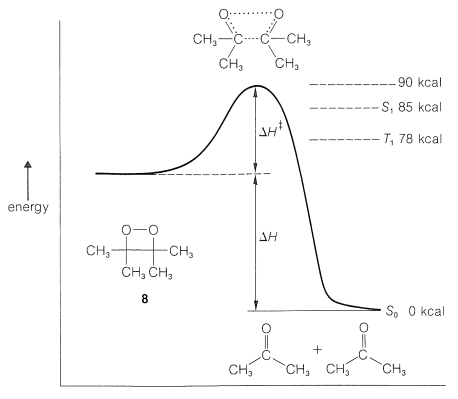
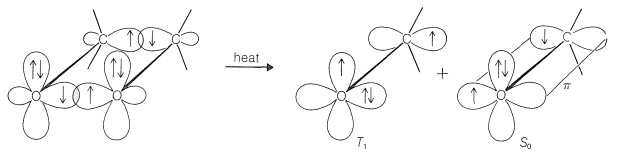
Of major interest is the identity of the excited state (singlet or triplet) produced by chemiluminescent reactions. Little is known about excited states produced chemically except in a few cases, as in Reaction 28-11. Here the chemiluminescence dissociation gives a ratio of triplet 2-propanone to excited singlet 2-propanone of 100:1. This is a surprising result because it means that spectroscopic selection rules of electron-spin conservation are not followed in this chemiexcitation. The reaction has generated a triplet state from a singlet state. How can this be? Some idea of what is involved can be obtained from Figure 28-7, in which we see that breaking of the two sigma \(\ce{C-C}\) and \(\ce{O-O}\) bonds gives directly one molecule of ground-state ketone (all spins paired) and one molecule of triplet ketone. In this process, the electrons associated with the orbitals on one of the oxygen atoms appear to interact in such a way as to interchange electrons between orbitals on the same atoms with a spin inversion. This is called spin-orbit coupling.
Bioluminescence
The emission of visible light by living organisms is a mysterious and fascinating phenomenon. The magical glow of the firefly and of certain plants and marine animals is a familiar sight and one that has stimulated man's curiosity and imagination for centuries. Despite intense interest in bioluminescence, it is only recently that substantial progress has been made in our understanding of how it occurs.
One of the earliest studies of bioluminescence was made by the French scientist R. Dubois toward the end of the nineteenth century. He demonstrated that bioluminescent organisms emitted light as a consequence of chemical change. He succeeded in isolating the active chemical from fireflies (luciferin) and the activating enzyme (luciferase, named by Dubois from the Latin lucifer, meaning light bearer). Luciferin and the enzyme in the presence of oxygen were found to reproduce the natural bioluminescence:
\[\text{luciferin} + \ce{O_2} \overset{\text{luciferase}}{\longrightarrow} \text{oxyluciferin} + h \nu\]
Further progress required elucidation of the structures of luciferin and its oxidation product. It turned out that there are several luciferins, depending on the organism. Firefly luciferin has the benzothiazol structure, \(9\); the luciferins from the marine crustacean Cypridina hilgendorfii and the sea pansy Renilla reformis have structures \(10\) and \(11\), respectively. Their oxidation products \(12\), \(13\), and \(14\) also are shown:
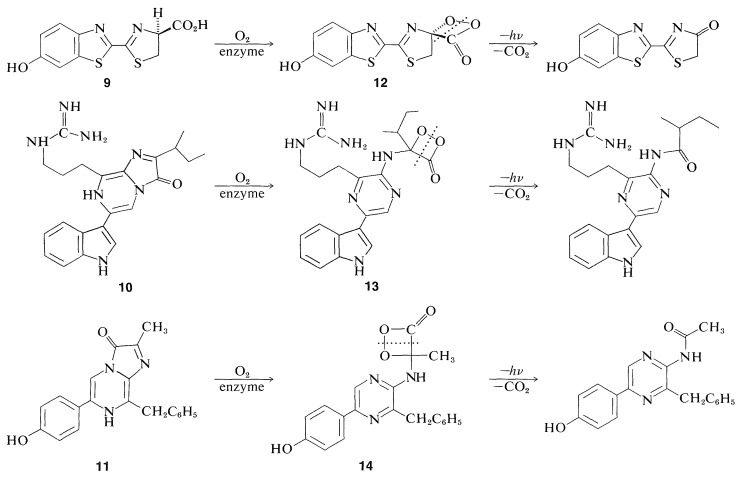
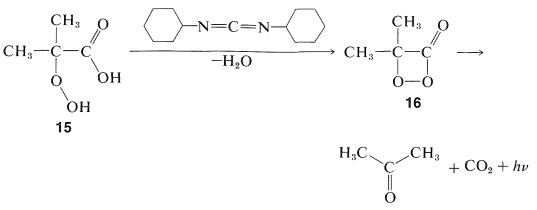
Although the luciferins \(9\)-\(11\) may not seem closely related, each appears to react with oxygen (at the direction of the appropriate enzyme) to give cyclic peroxylacetone intermediates \(12\)-\(14\). Luminescence is the consequence of the energetically favorable dissociation of the dioxacyclobutanone ring system to carbon dioxide and a carbonyl component. This mechanism is suggested by experiments with the peroxy acid, \(15\), which with \(\ce{N}\),\(\ce{N}\)-dicyclohexylcarbodiimide gives a very reactive compound presumed to be the peroxylactone, \(16\). This substance liberates \(\ce{CO_2}\) rapidly at room temperature with luminescence:
\(^4\)Production of two molecules of excited 2-propanone per molecule of \(8\) is not possible under the same conditions because this would correspond to a reaction with \(\Delta H^0\) of at least \(156 \: \text{kcal}\) above formation of two moles of ground-state ketone.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


