27.8: Field- and Chemical-Ionization Mass Spectroscopy
- Page ID
- 22390
As we mentioned in connection with our discussion of mass spectroscopy in Section 9-11, one problem with the practical application of mass spectra to structure analysis involving the production of ions by electron impact is that the \(\ce{M^+}\) peak may be very weak. In many situations we would like to have mass spectra with less intensive fragmentation than that obtainable by electron impact. There are two ways of achieving ion formation without imparting as much energy as by electron impact - in other words, "soft" rather than "hard" ionizations. Field ionization is one such method, in which ionization results from passing the molecules through an electric field of \(10^7\)-\(10^8 \: \text{V cm}^{-1}\). This may seem like a practically unattainable electric field. However, it can be achieved easily by impressing a potential of \(10^4\) volts across a pair of electrodes, one of which has a very sharp radius of curvature \(\left( \sim 10^{-5} \: \text{cm} \right)\), as can be achieved with a very fine metal point, very fine wire, or a sharp edge. Field ionization of a molecule differs from electron-impact ionization in that the electron normally is ejected from the molecule in its ground state. As a result, the parent ion \(\ce{M^+}\) peak is very strong, even for molecules for which the \(\ce{M^+}\) is virtually absent on electron impact.
Chemical ionization is, as might be expected from its name, more chemically interesting and is closely allied to ion cyclotron resonance, which will be discussed in the next section. The principle of chemical ionization is simple. The molecule to be studied is injected into the ionizing region of the mass spectrometer in the presence of \(0.5\)-\(1.5 \: \text{mm} \: \ce{Hg}\) pressure of a gas, usually methane. Electron impact causes ionization of the methane, which is present in relatively large concentration. The ionization products of methane then react with the compound to be analyzed and convert it to ions. The gas mixture then exits into a low-pressure zone \(\left( 10^{-4} \: \text{mm} \right)\) and the ions are analyzed according to m/e in the usual way.
What happens to \(\ce{CH_4}\) when it is bombarded with electrons at, say, \(1 \: \text{mm}\) pressure? The simplest reaction is formation of the \(\ce{M^+}\) ion from \(\ce{CH_4} + \ce{e^-} \rightarrow \ce{CH_4^+} + 2 \ce{e^-}\), but \(\ce{CH_3^+}\) and \(\ce{CH_2^+}\) also are produced by electron impact. If there is sufficient \(\ce{CH_4}\), a variety of rapid transformations take place between each of the ions produced by electron impact and the neutral \(\ce{CH_4}\). The principal ions formed are
Of these, the methonium cation, \(\ce{CH_5^+}\), is formed in largest amounts, the ethyl cation, \(\ce{C_2H_5^+}\), is next, and there is a smaller amount of the \(\ce{C_3H_5^+}\) cation (2-propenyl cation; Section 8-7B). These ions then react with the substance to be analyzed, thereby converting it into ions. Different reactions are possible, but it we have an unsaturated compound, call it \(\ce{RH}\), then
We then have a strong \(\left( \ce{M} - 1 \right)^+\) peak and weaker \(\left( \ce{M} + 29 \right)^+\) and \(\left( \ce{M} + 41 \right)^+\) peaks. The larger cations probably are similar to those formed in cationic polymerization (Section 10-8B), whereas formation of the \(\left( \ce{M} - 1 \right)^+\) cation corresponds to the hydrogen-transfer reaction discussed in Section 10-9.
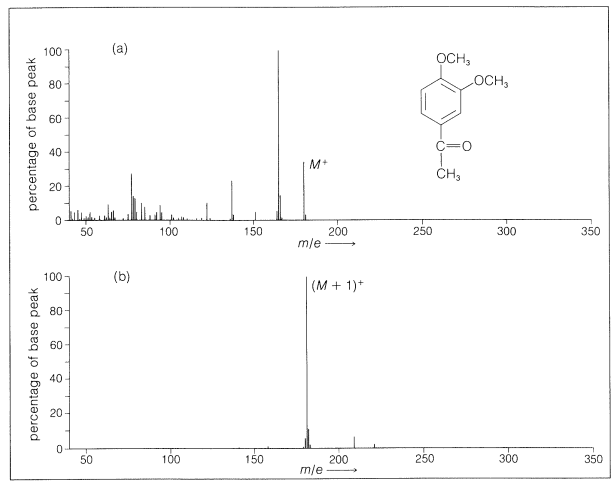
With many compounds there is little fragmentation on chemical ionization. An example of a comparison of the spectra resulting from electron impact and chemical ionization is given in Figure 27-13. The simplicity of the spectra makes chemical-ionization mass spectroscopy especially useful for continuous analysis of the effluent from gas-liquid chromatographic columns (Section 9-2).
A problem with all mass spectroscopy of large molecules is how to get them into the vapor phase so that they can be ionized and their fragmentation patterns determined. Simple heating may cause excessive degradation and formation of ions not corresponding to the desired substance. Two useful methods that involve only intense short-term local heating of the sample appear to have promise in this connection. One method uses a burst from a powerful infrared laser to volatilize part of the sample, and the other uses bombardment by heavy and energetic particles from fission of californium-252 nuclei to raise the local temperature of the sample to about \(10,000^\text{o}\). The latter technique both volatilizes and ionizes the sample molecules.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


