25.6: Synthesis of α-Amino Acids
- Page ID
- 22362
Many of the types of reactions that are useful for the preparation of amino acids have been discussed previously in connection with separate syntheses of carboxylic acids (Chapter 18) and amino compounds (Chapter 23). Examples include the \(S_\text{N}2\) displacement of halogen from \(\alpha\)-halo acids by ammonia,
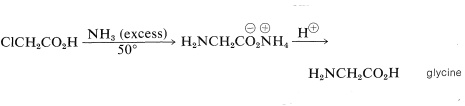
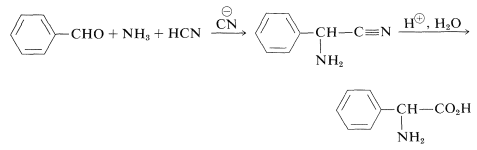
and the Strecker synthesis, which, in its first step, bears a close relationship to cyanohydrin formation (Section 16-4A):
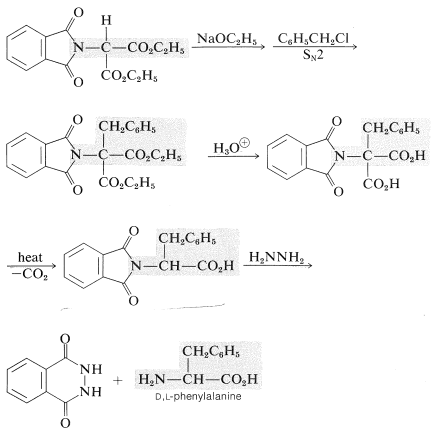
Other general synthetic methods introduce the \(\alpha\)-amino acid grouping, \(\ce{H_2N-CH-CO_2H}\), by way of enolate anions. Two selected examples follow. Notice that in each a carbanion is generated and alkylated. Also the \(\ce{H_2N}-\) group is introduced as a protected amide or imide group.
1. phthalimidomalonic ester synthesis
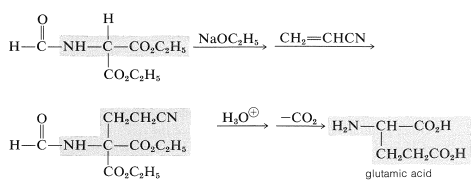
2. \(\ce{N}\)-formylaminomalonic ester synthesis
With those amino acids that are very soluble in water, it usually is necessary to isolate the product either by evaporation of an aqueous solution or by precipitation induced by addition of an organic solvent like alcohol. Difficulty may be encountered in obtaining a pure product when inorganic salts are coproducts of the synthesis. The best general method for removal of inorganic salts involves passage of the solutions through columns of suitable ion-exchange resins (Section 25-4C).
The products of laboratory syntheses, starting with achiral reagents, are of course racemic \(\alpha\)-amino acids. To obtain the natural amino acids, the \(D\),\(L\) mixtures must be resolved (Section 19-3).
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


