23.8: Amines as Acids
- Page ID
- 22343
Primary and secondary amines are very weak acids. The lithium salts of such amines can be prepared in ether solution by treatment of the amine with phenyllithium:
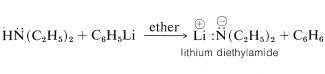
The lithium salt of \(\ce{N}\)-ethylethanamine (diethylamine) is called lithium diethylamide,\(^4\) but this nomenclature can lead to confusion with compounds of the type \(\ce{RCO_2NH_2}\), which are derived from carboxylic acids and also are called amides. We choose to avoid using the name "alkali amide" for \(\ce{RN} \overset{\ominus}{\ce{H}} \overset{\oplus}{\ce{Li}}\) and accordingly will refer to them as metal salts of the parent amine.
Alkanamines have acid strengths corresponding to \(K_a\) values of about \(10^{-33}\), which means that their conjugate bases are powerfully basic reagents. Therefore they are very effective in causing elimination reactions by the \(E2\) mechanism (Section 8-8) and aromatic substitution by the aryne mechanism (Section 14-6C). The following example illustrates this property in a useful synthesis of a benzenamine from bromobenzene:
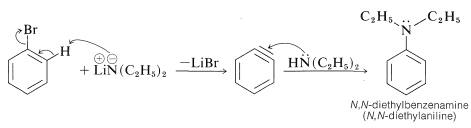
Salts of alkanamines also are useful for generating enolate salts of carbonyl compounds (Sections 17-4A and 18-8C).
\(^4\)The system used here names these salts as substitution products of \(\ce{NH_2^-}\). Clearly, to give \(\ce{LiN(C_2H_5)_2}\) the name "lithium \(\ce{N}\)-ethylethanamide" would be totally incorrect because \(\ce{N}\)-ethylethanamide is \(\ce{CH_3CONHC_2H_5}\). Perhaps a better name would be lithium diethylazanide or \(\ce{N}\),\(\ce{N}\)-diethylaminolithium.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


