23.2: Naturally Occurring Amines
- Page ID
- 22337
A large and widespread class of naturally occurring amines is known as alkaloids. These are basic organic nitrogen compounds, mostly of plant origin. The structures of the plant alkaloids are extraordinarily complex, yet they are related to the simple amines in being weak nitrogen bases. In fact, the first investigator to isolate an alkaloid in pure form was F. W. A. Sertürner who, in 1816, described morphine (Figure 23-1) as basic, salt-forming, and ammonia-like. He used the term "organic alkali" from which is derived the name alkaloid.
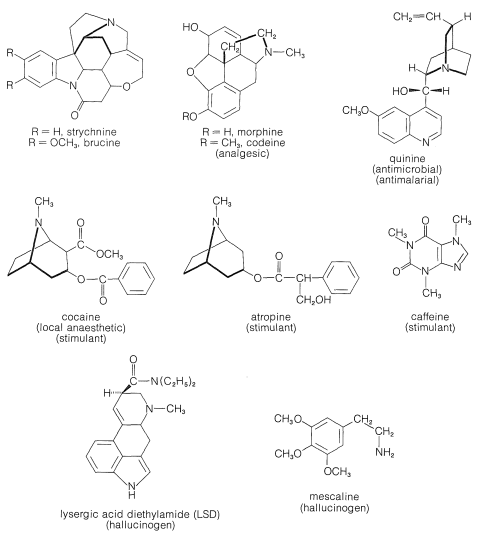
The structures of some of the better known plant alkaloids are shown in Figure 23-1. You will recognize some of them by name even if you have never seen their structures before. Many of the alkaloids are polycyclic structures and have other functional groups in addition to basic nitrogen. You will see that the nitrogens of alkaloids frequently are tertiary amine functions.
All of the alkaloids shown in Figure 23-1 are substances with very pronounced physiological action. Indeed, alkaloids in general have been used and abused for centuries as medicinals, drugs, and poisons. However, only in this century have their structures become known, and we are still a long way from understanding the chemistry that leads to their pronounced physiological effects. It is not even understood what function, if any, these compounds have in the host plant.
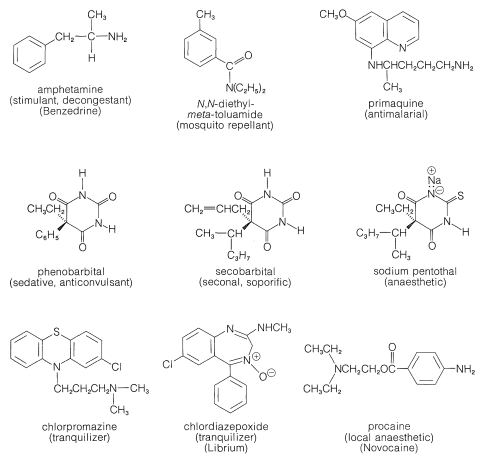
As you can see from Figure 23-1, alkaloids include compounds that may be classified as antimicrobial (quinine), as analgesics (morphine, codeine), as hallucinogens (mescaline, LSD), as stimulants (cocaine, atropine, caffeine), as topical anaesthetics (cocaine). With the possible exception of caffeine, all may be described as potentially poisonous enough to warrant great care in their use. Although some of these compounds are used as natural medicinals, an entire industry has developed in an effort to produce synthetic analogs with similar, but safer, medicinal properties. Some of the better known of these synthetic drugs are shown in Figure 23-2. They include a group of narcotic substances known as barbiturates, which are used widely as sedatives, anticonvulsants, and sleep-inducing drugs. Several representative nitrogen-containing tranquilizing drugs, synthetic stimulants, and antibiotics also are shown.
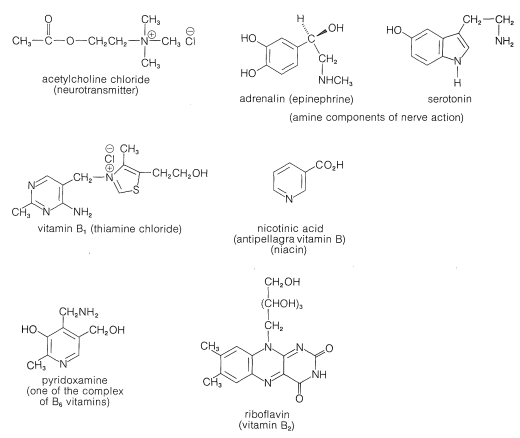
Basic nitrogen compounds similar to the plant alkaloids also occur in animals, although the description animal alkaloid seldom is used. Certain amines and ammonium compounds play key roles in the function of the central nervous system (Figure 23-3) and the balance of amines in the brain is critical for normal brain functioning. Also, many essential vitamins and hormones are basic nitrogen compounds. Nitrogen bases also are vital constituents of nucleic acid polymers (DNA and RNA) and of proteins (Chapter 25).
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


