23.1: Amines Compared with Alcohols
- Page ID
- 22336
As you read the chapter you will realize a similarity between the chemistry of amines and the chemistry of alcohols, which we discussed in Chapter 15. Primary amines \(\left( \ce{RNH_2} \right)\) and secondary amines \(\left( \ce{R_2NH} \right)\) are much weaker acids than alcohols \(\left( \ce{ROH} \right)\) and form strongly basic anions:
Acids
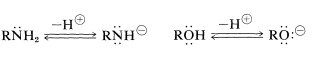
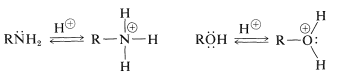
Amines, like alcohols, have nonbonding electrons that impart basic and nucleophilic properties:
Bases
Nucleophiles
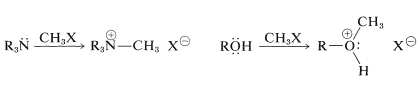
Also, amines and alcohols both can behave as carbon electrophiles under appropriate reaction conditions such that cleavage of \(\ce{C-N}\) and \(\ce{C-O}\) bonds occurs in the sense \(\overset{\delta \oplus}{\ce{C}} \vdots \overset{\delta \ominus}{\ce{N}}\) and \(\overset{\delta \oplus}{\ce{C}} \vdots \overset{\delta \ominus}{\ce{O}}\). However, because \(\ce{-NH_2}\) and \(\ce{-OH}\) both are poor leaving groups, each must be suitably activated to make this kind of reaction possible (see Section 8-7C). The \(\ce{OH}\) group can be activated by addition of a proton or conversion to a sulfonate ester, \(\ce{RO_3SR'}\), but these processes generally are ineffective for \(\ce{RNH_2}\). The most effective activation for \(\ce{RNH_2}\) is through conversion with nitrous acid, \(\ce{HONO}\), to \(\ce{R}- \overset{\oplus}{\ce{N}} \equiv \ce{N}\); then \(\ce{N_2}\) is the leaving group (this reaction is described in more detail in Section 23-10A):
\[\ce{R-OH} \overset{\ce{HBr}}{\longrightarrow} \ce{R-Br} + \ce{H_2O}\]
There is, though a major difference in the way that amines and alcohols behave toward oxidizing agents. Amines genearlly show more complex behavior on oxidation because, as we shall see, nitrogen has a larger number of stable oxidation states than oxygen.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


