22.8: Substitution Reactions of Polynuclear Aromatic Hydrocarbons
- Page ID
- 22330
Although naphthalene, phenanthrene, and anthracene resemble benzene in many respects, they are more reactive than benzene in both substitution and addition reactions. This increased reactivity is expected on theoretical grounds because quantum-mechanical calculations show that the net loss in stabilization energy for the first step in electrophilic substitution or addition decreases progressively from benzene to anthracene; therefore the reactivity in substitution and addition reactions should increase from benzene to anthracene.
In considering the properties of the polynuclear hydrocarbons relative to benzene, it is important to recognize that we neither expect nor find that all the carbon-carbon bonds in polynuclear hydrocarbons are alike or correspond to benzene bonds in being halfway between single and double bonds.
The 1,2 bonds in both naphthalene and antracene are in fact shorter than the other ring bonds, whereas the 9,10 bond in phenanthrene closely resembles an alkene double bond in both its length and chemical reactivity.
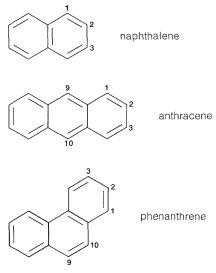
Naphthalene
Orientation in the substitution of naphthalene can be complex, although the 1 position is the most reactive. Some examples follow.
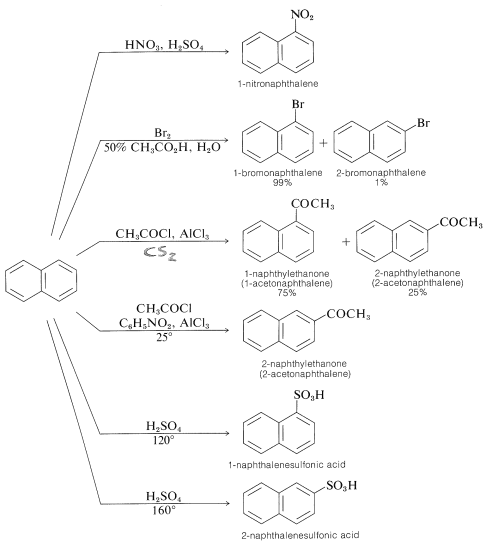
Sometimes, small changes in the reagents and conditions change the pattern of orientation. One example is sulfonation, in which the orientation changes with reaction temperature. Another example is Friedel-Crafts acylation; in carbon disulfide the major product is the 1-isomer, whereas in nitrobenzene the major product is the 2-isomer.
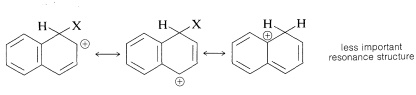
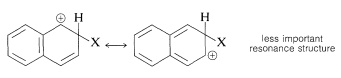
Substitution usually occurs more readily at the 1 position than at the 2 position because the intermediate for 1-substitution is more stable than that for 2-substitution. The reason is that the most favorable resonance structures for either intermediate are those that have one fully aromatic ring. We can see that 1-substitution is more favorable because the positive charge can be distributed over two positions, leaving one aromatic ring unchanged. Only one resonance structure is possible for the 2-substitution intermediate that retains a benzenoid-bond arrangement for one of the rings.
1-substitution
2-substitution
Phenanthrene
The reactions of the higher hydrocarbons with electrophilic reagents are more complex than of naphthalene. For example, phenanthrene can be nitrated and sulfonated, and the products are mixtures of 1-, 2-, 3-, 4-, and 9-substituted phenanthrenes:

However, the 9,10 bond in phenanthrene is quite reactive; in fact is is almost as reactive as an alkene double bond. Addition therefore occurs fairly readily; halogenation can give both 9,10-addition and 9-substitution products by the following scheme:
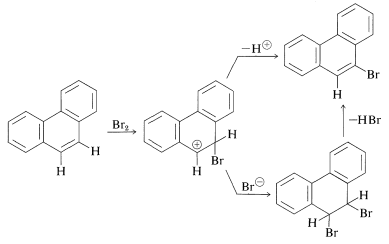
Anthracene
Anthracene is even more reactive than phenanthrene and has a greater tendency to add at the 9,10 positions than to substituted. However, the addition products of nitration and halogenation readily undergo elimination to form the 9-substitution products:
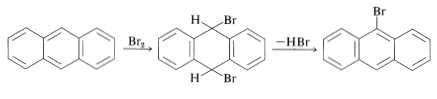
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


