18.6: Reactions at the \(\alpha\) Carbons of Carboxylic Acids
- Page ID
- 22291
Halogenation
Bromine reacts smoothly with carboxylic acids in the presence of small quantities of phosphorus to form alpha-bromocarboxylic acids (Hell-Volhard-Zelinsky reaction):
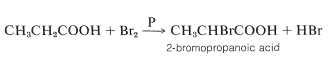
The reaction is slow in the absence of phosphorus, whose function appears to be to form phosphorus tribromide, which then reacts with the acid to give the acyl bromide:
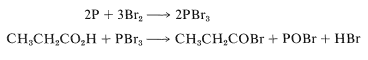
Formation of the acyl bromide speeds up the reaction because acid-catalyzed enolization of the acyl bromide occurs much more readily than enolization of the parent acid. Bromine probably reacts with the enol of the acyl bromide in the same way as it reacts with the enols of ketones (Section 17-2A).
The final step is the formation of the \(\alpha\)-bromo acid by bromine exchange between the \(\alpha\)-bromoacyl bromide and the parent acid; the acyl bromide, which is necessary for continued reaction, is thus regenerated:
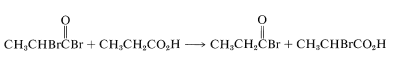
This bromination reaction results exclusively in alpha substitution and therefore is limited to carboxylic acids with \(\alpha\) hydrogens. Chlorine with a trace of phosphorus reacts similarly but with less overall specificity, because concurrent free-radical chlorination can occur at all positions along the chain (as in hydrocarbon halogenation; see Section 4-6A).
Substitution Reactions of \(\alpha\)-Haloalkanoic Acids
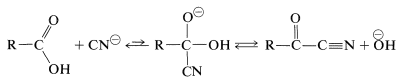
The halogen of an \(\alpha\)-haloalkanoic acid is replaced readily by nucleophilic reagents such as \(\ce{CN}^\ominus\), \(\ce{OH}^\ominus\), \(\ce{I}^\ominus\), and \(\ce{NH_3}\). Thus a variety of \(\alpha\)-substituted carboxylic aids may be prepared by reactions that are analogous to \(S_\text{N}2\) substitution of alkyl halides:
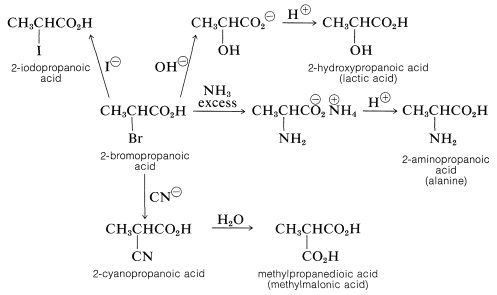
Facile \(S_\text{N}2\) substitution reactions of halogens are expected from the electron-attracting characteristics of the neighboring carbonyl function, which should make the transition state for attack by a nucleophilic reagent more favorable:
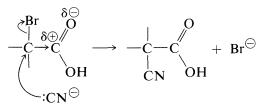
Perhaps it may seem surprising that the carboxyl carbon is not attacked by the nucleophilic agents, because we have stressed earlier the susceptibility of carbonyl groups to nucleophilic reagents. No stable product results, however, from addition to the carbonyl group by the type of reagents considered here. Thus with cyanide ion the equilibrium constant for addition is unfavorable because of the associated loss of stabilization energy of the carboxyl group.
The \(S_\text{N}1\) reactivity of \(\alpha\)-haloalkanoic acids is particularly low. This is reasonable because formation of a cationic center at the \(\alpha\) carbon should be difficult, because of the positive character of the carbonyl carbon. Furthermore, little, if any, help could be expected through electron delocalization because the corresponding valence-bond structure has a positive, single-bonded oxygen:
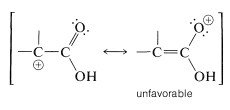
Similar considerations apply to the \(S_\text{N}1\) and \(S_\text{N}2\) reactions of \(\alpha\)-halo aldehydes and \(\alpha\)-halo ketones (Section 17-2C).
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


