18.1: Prelude to Carboxylic Acids and Their Derivatives
- Page ID
- 79330
Almost all of the basic types of reactions now have been covered: addition, elimination, substitution, and rearrangement by polar, radical, and concerted mechanisms. Indeed, if you have been looking for similarities, you will have seen that most of the reactions discussed in the preceding three chapters are variations on basic types we have discussed earlier. Furthermore, most of the basic structural effects that determine chemical reactivity also have been covered in previous chapters: bond energies, steric hindrance, electronegativity, electron delocalization, hydrogen bonding, solvation, and conformational influences.
You might well ask what is left. The answer is, a great deal - but now we will be concerned mostly with putting concepts together, moving from the simple to the complex. For example, in this chapter we will be trying to understand the ways that carboxylic acids, which possess the \(\ce{-COOH}\) functional group, are similar to and different from alcohols, which have the \(\ce{-OH}\) group, and aldehydes and ketones, which have \(\ce{C=O}\) bonds.
Subsequently we will look at acids that also possess \(\ce{OH}\) or \(\ce{NH_2}\) substituent groups (or both) and develop a rationale for the behavior of these combinations in terms of effects we already have discussed. Insofar as possible, you should try to do this yourself whenever you encounter a substance with a new set of combinations or functional groups on its molecules. You often will be in error (as many experts will be), because even in you take account of all of the structural effects, as well as the possible reactions or interactions, the overall result of these frequently is very difficult to judge in advance. In one case, steric hindrance may dominate, in another, electron delocalization, and so on. Still, trying to assess the effects and possible reactions leads to understanding and recognition of what the alternatives are, even if the resultant of them is difficult to assess.\(^1\) Continuing study can be expected to develop an instinct for what is "good" chemistry and what is not.
We have described previously the acidic properties of several types of compounds: alkynes, alkenes, and alkanes (Sections 11-8 and 13-5B); halides (Section 14-7B); alcohols (Section 15-4A); and carbonyl compounds (Section 17-1A). Now we come to compounds that we actually call acids - the carboxylic acids, \(\ce{RCO_2H}\). Are these acids different in kind, or only in degree, from other acidic compounds discussed before? This is not a simple question and deserves some thought. In the most widely used sense, acids are proton donors but, as we have seen, their abilities to donate a proton to water vary over an enormous range: \(\ce{CH_4}\) has a \(K_a\) of \(< 10^{-40}\), whereas \(\ce{HI}\) has a \(K_a\) of \(\sim 10^9\). This represents a difference in ionization energies of more than \(70 \: \text{kcal mol}^{-1}\). The differences in \(K_a\) are only differences in degree, because examples are available of acids with \(K_a\) values in all parts of the range of \(K_a\) values. An important difference in kind was mentioned in Section 17-1B, namely, that acids with the same \(K_a\) values can differ greatly in the rates at which they give up a proton to a given base, such as water. Carbon acids, in which the proton comes from a \(\ce{C-H}\) bond, may react more than \(10^{10}\) times slower than an oxygen acid with the same \(K_a\) in which the proton is give up from an \(\ce{O-H}\) bond.
Tradition reserves the use of the name "acid" for substances that transfer protons measurably to water. Thus the carboxylic acids stand out from alkynes, halides, alcohols, and simple aldehydes and ketones in giving water solutions that are "acidic" to indicator papers or pH meters as the result of proton transfers from the carboxylic groups:
\[\ce{RCO_2H} + \ce{H_2O} \rightleftharpoons \ce{RCO_2^-} + \ce{H_3O^+}\]
Even so, carboxylic acids are not very strong acids and, in a \(1 \: \text{M}\) water solution, a typical carboxylic acid is converted to ions to the extent of only about \(0.5\%\).
The nomenclature of carboxylic acids and their derivatives was discussed in Section 7-6. Many carboxylic acids have trivial names and often are referred to as "fatty acids". This term applies best to the naturally occurring straight-chain saturated and unsaturated aliphatic acids, which, as esters, are constituents of the fats, waxes, and oils of plants and animals. The most abundant of these fatty acids are palmitic, stearic, oleic, and linoleic acids. They occur as glycerides, which are esters of the trihydric alcohol, 1,2,3-propanetriol (glycerol):
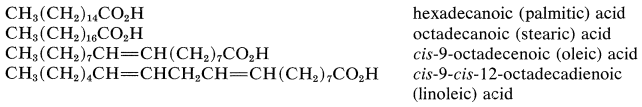
Fats or glycerides belong to a class of biomolecules known as lipids. The traditional definition of a lipid is a water-insoluble organic compound that can be extracted from cells and tissues by nonpolar solvents (chloroform, ether, benzene). Compounds that meet this definition are substantially hydrocarbonlike, although they may differ widely in structure. They include not only esters of long-chain fatty acids but steroids (Section 30-4), terpenes (Section 30-3), and prostaglandins (Section 30-7). These nonpolar substances serve a variety of different biological functions, but lipids derived from fatty acids are most important for energy storage (Sections 18-8F and 20-10) and as components of membranes. Phosphoglycerides represent an important class of membrane lipids derived from glycerol. The hydroxyl groups of glycerol are esterified with two fatty-acid chains and one phosphate-ester residue. One of the phosphate-ester groups carries a highly polar \(\ce{-N(CH_3)_2^+}\) group, the importance of which is indicated in Section 18-2F:
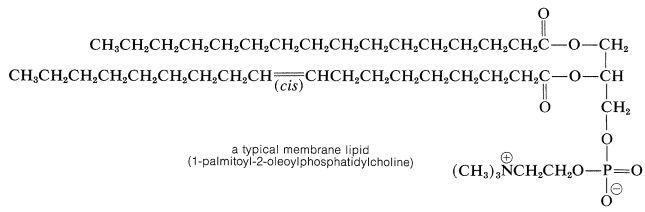
Hydrolysis of fats with alkali (e.g., sodium hydroxide) yields salts of the fatty acids, and those of the alkali metals, such as sodium or potassium, are useful as soaps:
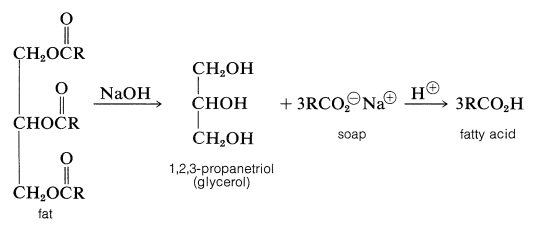
The properties of salts of long-chain carboxylic acids that make them useful as soaps will be discussed in Section 18-2F.
General methods for the preparation of carboxylic acids are summarized in Table 18-5, at the end of the chapter.
\(^1\)The major problem with assessing the resultant to be expected from opposing factors in chemical reactions is that relatively small energy differences can cause great differences in which product is favored. For an equilibrium such as \(\ce{A} \rightleftharpoons \ce{B}\) at \(25^\text{o} \text{C}\), a \(5.5 \: \text{kcal mol}^{-1}\) change in \(\Delta G^0\) (Section 4-4A) can cause the equilibrium to shift from \(99\%\) in favor of \(\ce{A}\) to \(99\%\) in favor of \(\ce{B}\).
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


