17.1: Prelude to Enols and Enolate Anions, Unsaturated, and Polycarbonyl Compounds
- Page ID
- 79329
Some of the most useful reactions of carbonyl compounds involve carbon-hydrogen bonds adjacent to the carbonyl group. Such reactions, which can be regarded as the backbone of much synthetic organic chemistry, usually result in the replacement of the hydrogen by some other atom or group, as in the sequence \(\ce{H-C-C=O} \rightarrow \ce{X-C-C=O}\). The important examples we will consider in this chapter are halogenation, alkylation, and aldol reactions of aldehydes and ketones, illustrated here for 2-propanone:
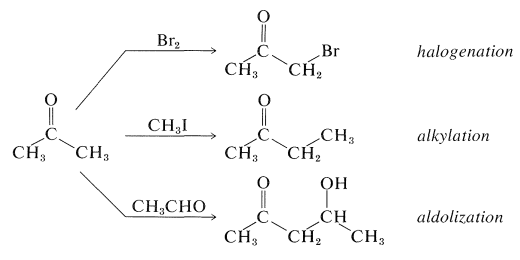
Although these reactions lead to many diverse products depending on the reagents and conditions, they have one feature in common - they proceed by way of the enol or the enolate anion of the parent carbonyl compound:
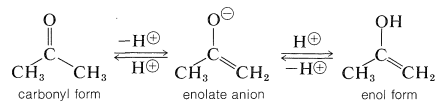
Therefore, to understand the nature of these reactions we first must understand the conditions that convert aldehydes and ketones to their enol forms or the anions of those enol forms.
Contributors
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


