16.3: Physical Properties
- Page ID
- 22270
The polarity of the carbonyl group is manifest in the physical properties of carbonyl compounds. Boiling points for the lower members of a series of aldehydes and ketones are \(50\)-\(80^\text{o}\) higher than for hydrocarbons of the same molecular weight; this may be seen by comparing the data of Table 16-2 (physical properties of aldehydes and ketones) with those in Table 4-1 (physical properties of alkanes).
Table 16-2: Physical Properties of Aldehydes and Ketones
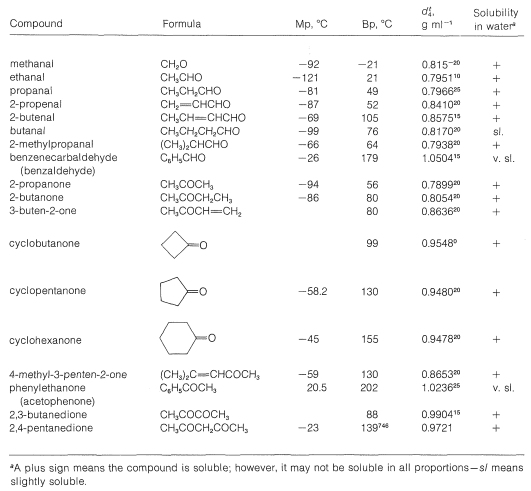
The water solubility of the lower-molecular-weight aldehydes and ketones is pronounced (see Table 16-2). This is to be expected for most carbonyl compounds of low molecular weight and is the consequence of hydrogen-bonding between the water and the electronegative oxygen of the carbonyl group:

Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


