15.11: Types and Reactions of Simple Ethers
- Page ID
- 21999
Substitution of the hydroxyl hydrogens of alcohols by hydrocarbon groups gives compounds known as ethers. These compounds may be classified further as open-chain, cyclic, saturated, unsaturated, aromatic, and so on. For the naming of ethers, see Sections 7-3 and 15-11A.
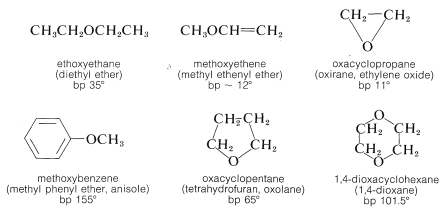
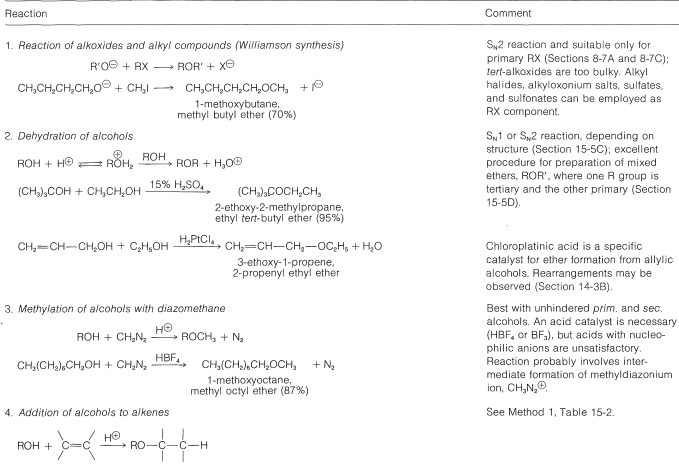
The most generally useful methods of preparing ethers already have been discussed (Sections 8-7C, 8-7E, 15-4C, and 15-5C). These and some additional special procedures are summarized in Table 15-4.
Table 15-4: General Methods of Preparation of Ethers
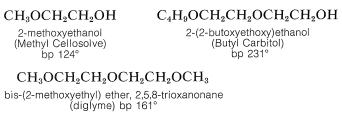
In general, ethers are low on the scale of chemical reactivity because the carbon-oxygen bond is not cleaved readily. For this reason ethers frequently are employed as inert solvents in organic synthesis. Particularly important in this connection are diethyl ether, diisopropyl ether, tetrahydrofuran, and 1,4-dioxane. The mono- and dialkyl ethers of 1,2-ethanediol, 3-oxa-1,5-pentanediol, and related substances are useful high-boiling solvents. Unfortunately, their trade names are not very rational. Abbreviated names are in common use, such as “polyglymes,” “Cellosolves,” and “Carbitols.” For reference, Cellosolves are monoalkyl ethers of 1,2-ethanediol; Carbitols are monoalkyl ethers of 3-oxa-1,5-pentanediol; polyglymes are dimethyl ethers of 3-oxa-1,5-pentanediol or 3,6-dioxa-1,8-octanediol and are called diglyme and triglyme, respectively.
The spectroscopic properties of ethers are unexceptional. Like alcohols, they have no electronic absorption beyond \(185 \: \text{nm}\); the important infrared bands are the \(\ce{C-O}\) stretching vibrations in the region \(1000\)-\(1230 \: \text{cm}^{-1}\); their proton nmr spectra show deshielding of the alpha hydrogens by the ether oxygen \(\left( \delta_{HC_{\alpha}OC} \sim 3.4 \: \text{ppm} \right)\). The mass spectra of ethers and alcohols are very similar and give abundant ions of the type 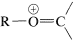 (\(\ce{R} = \ce{H}\) or alkyl) by \(\alpha\)-cleavage (see Section 15-2).
(\(\ce{R} = \ce{H}\) or alkyl) by \(\alpha\)-cleavage (see Section 15-2).
Unlike alcohols, ethers are not acidic and usually do not react with bases. However, exceptionally strong basic reagents, particularly certain alkali-metal alkyls, will react destructively with many ethers:
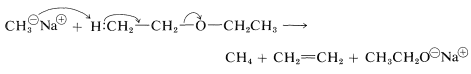
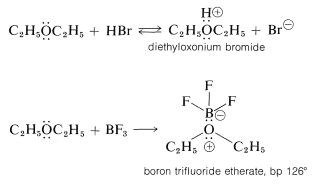
Ethers, like alcohols, are weakly basic and are converted to highly reactive salts by strong acids (e.g., \(\ce{H_2SO_4}\), \(\ce{HClO_4}\), and \(\ce{HBr}\)) and to relatively stable coordination complexes with Lewis acids (e.g., \(\ce{BF_3}\) and \(\ce{RMgX}\)):
Dimethyl ether is converted to trimethyloxonium fluoroborate by the combination of boron trifluoride and methyl fluoride:
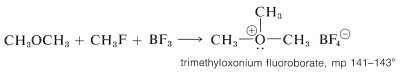
Both trimethyl- and triethyloxonium salts are fairly stable and can be isolated as crystalline solids. They are prepared more conveniently from the appropriate boron trifluoride etherate and chloromethyloxacyclopropane (epichlorohydrin).
Trialkyloxonium ions are much more susceptible to nucleophilic displacement reactions than are neutral ether molecules. The reason is that \(\ce{ROR}\) is a better leaving group than \(\ce{RO}^\ominus\). In fact, trimethyloxonium salts are among the most effective methylating reagents known:
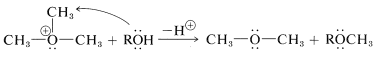
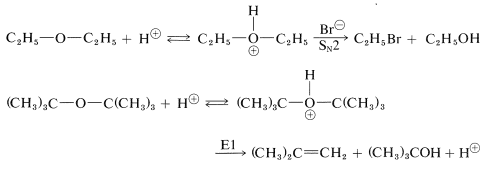
Ethers can be cleaved under strongly acidic conditions by intermediate formation of dialkyloxonium salts. Hydrobromic and hydroiodic acids are especially useful for ether cleavage because both are strong acids and their anions are good nucleophiles. Tertiary alkyl ethers are very easily cleaved by acid reagents:
Ethers are susceptible to attack by halogen atoms and radicals, and for this reason they are not good solvents for radical reactions. In fact, ethers are potentially hazardous chemicals, because in the presence of atmospheric oxygen radical-chain formation of peroxides occurs, and peroxides are unstable, explosion-prone compounds. This process is called autoxidation and occurs not only with ethers but with many aldehydes and hydrocarbons. The reaction may be generalized in terms of the following steps involving initiation (1), propagation (2 and 3), and termination (4).

The initiation and termination steps can occur in a variety of ways but it is the chain-carrying steps, 2 and 3, that effect the overall destruction of the compound. Commonly used ethers such as diethyl ether, diisopropyl ether, tetrahydrofuran, and 1,4-dioxane often become seriously contaminated with peroxides formed by autoxidation on prolonged storage and exposure to air and light. Purification of ethers frequently is necessary before use, and caution always should be exercised in the last stages of distilling them, because the distillation residues may contain dangerously high concentrations of explosive peroxides.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


