15.1: Prelude to Alcohols and Ethers
- Page ID
- 78385
The physical, chemical and spectroscopic properties of alcohols are relative to it’s chemical structures. Alcohols are compounds of the general formula ROH, where R is any alkyl or substituted alkyl group. The hydroxyl group (OH groups) is the characteristic functional group of alcohols and is one of the most important functional groups of naturally occurring organic molecules. All carbohydrates and their derivatives, including nucleic acids, have hydroxyl groups. Some amino acids, most steroids, many terpenes, and plant pigments have hydroxyl groups. These substances serve many diverse purposes for the support and maintenance of life. One extreme example is the potent toxin tetrodotoxin, which is isolated from puffer fish and has obvious use for defense against predators. This compound has special biochemical interest, having six different hydroxylic functions arranged on a cagelike structure:
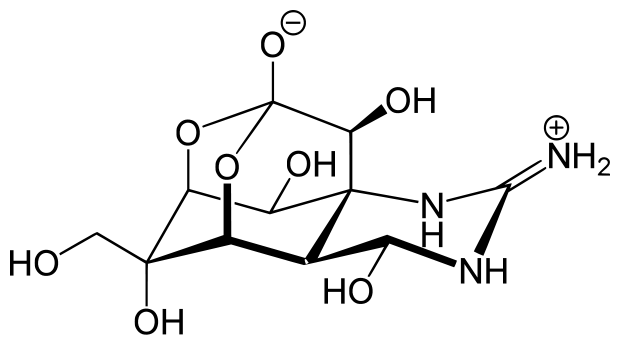
).
On the more practical side, vast quantities of simple alcohols —methanol, ethanol, 2-propanol, 1-butanol —and many ethers are made from petroleum-derived hydrocarbons. These alcohols are widely used as solvents and as intermediates for the synthesis of more complex substances.
The reactions involving the hydrogens of alcoholic OH groups are expected to be similar to those of water, HOH, the simplest hydroxylic compound. Alcohols, ROH, can be regarded in this respect as substitution products of water. However, with alcohols we shall be interested not only in reactions that proceed at the O-H bond but also with processes that result in cleavage of the C-O bond, or changes in the organic group R.
The simple ethers, ROR, do not have O-H bonds, and most of their reactions are limited to the substituent groups. The chemistry of ethers, therefore, is less varied than that of alcohols. This fact is turned to advantage in the widespread use of ethers as solvents for a variety of organic reactions, as we already have seen for Grignard reagents. Nonetheless, cyclic ethers with small rings show enhanced reactivity because of ring strain and, for this reason, are valuable intermediates in organic synthesis.
Before turning to the specific chemistry of alcohols and ethers, we remind you that the naming alcohols and ethers is summarized in naming alcohols, phenols and Naming Ethers.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


