13.9: Construction of Ring Systems by Cycloaddition
- Page ID
- 22057
Another example of a synthesis problem makes use of the cycloaddition reactions discussed here. Consider the synthesis of bicyclo[2.2.1]heptane, \(9\), from compounds with fewer carbons.
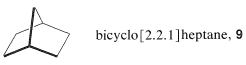
Whenever a ring has to be constructed, you should consider the possibility of cycloaddition reactions, especially [4 + 2] cycloaddition by the Diels-Alder reaction. A first glance at \(9\), written in the usual sawhorse-perspective formula, might lead to overlooking the possibility of constructing the skeleton by [4 + 2] addition, because the compound seems only to be made up of five-membered rings. If the structure is rewritten as \(10\), the six-membered ring stands out much more clearly:
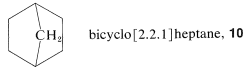
If we now try to divide the six-membered ring into [2] and [4] fragments, we find that there are only two different ways this can be done:
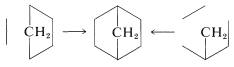
The left division corresponds to a simple [4 + 2] cycloaddition, whereas the right division corresponds to a complex reaction involving formation of three ring bonds at once. Actual Diels-Alder reactions require diene and dienophile starting materials, and two possibilities, using 1,3-cyclopentadiene as the diene and ethene or ethyne as dienophile, follow:
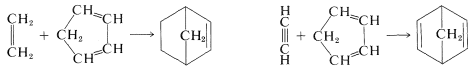
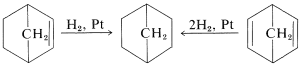
Either of the products can be converted to bicyclo[2.2.1]heptane by hydrogenation (Table 13-5):
Neither ethene nor ethyne is a very good dienophile but [4 + 2] cycloadditions of either with 1,3-cyclopentadiene go well at temperatures of \(160\)-\(180^\text{o}\) because 1,3-cyclopentadiene is a very reactive diene. Achieving the overall result of addition of ethene or ethyne to a less reactive diene could necessitate a synthetic sequence wherein one of the reactive dienophiles listed in Table 13-1 is used to introduce the desired two carbons, and the activating groups are subsequently removed. An example follows:
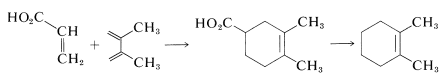
Reactions that can be used to remove a \(\ce{-CO_2H}\) group will be discussed in Chapter 18.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


