12.7: Cycloalkenes and Cycloalkanes
- Page ID
- 22077
The \(\ce{C-C=C}\) angle in alkenes normally is about \(122^\text{o}\), which is \(10^\text{o}\) larger than the normal \(\ce{C-C-C}\) angle in cycloalkanes. This means that we would expect about \(20^\text{o}\) more angle strain in small-ring cycloalkenes than in the cycloalkanes with the same numbers of carbons in the ring. Comparison of the data for cycloalkenes in Table 12-5 and for cycloalkanes in Table 12-3 reveals that this expectation is realized for cyclopropene, but is less conspicuous for cyclobutene and cyclopentene. The reason for this is not clear, but may be connected in part with the \(\ce{C-H}\) bond strengths (see Section 12-4B).
Cyclopropene has rather exceptional properties compared to the other cycloalkenes. It is quite unstable and the liquid polymerizes spontaneously although slowly, even at \(-80^\text{o}\). This substance, unlike other alkenes, reacts rapidly with iodine and behaves like an alkyne in that one of its double-bond hydrogens is replaced in silver-ammonia solution to yield an alkynide-like silver complex.
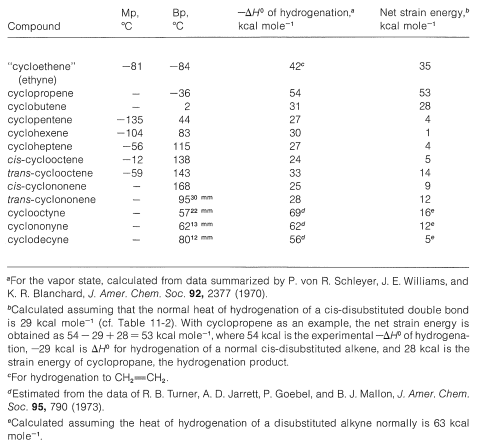
Table 12-5: Properties of Some Cycloalkenes and Cycloalkynes
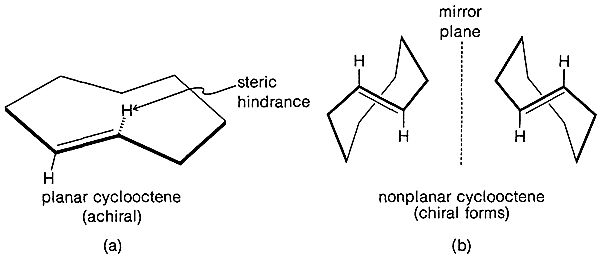
The \(\ce{C-C=C}\) bond angles in alkynes normally are \(180^\text{o}\) and the angle strain involved in making a small-ring cycloalkyne, such as cyclopropyne, apparently is prohibitive. The smallest reasonably stable member of the series is cyclooctyne, and its properties, along with those of some higher homologs, are shown in Table 12-5. Strong evidence has been adduced for the existence of cyclopentyne, cyclohexyne, and cycloheptyne as unstable reaction intermediates.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


