12.2: Spectroscopic Properties of Cyclohexanes
- Page ID
- 22086
The spectroscopic properties of cycloalkanes are considerably similar to those of alkanes. We mentioned previously the main features of their infrared spectra (Section 9-7D), and that their lack of ultraviolet absorption at wavelengths down to 200 nm makes them useful solvents for the determination of ultraviolet spectra of other substances (Section 9-9B).
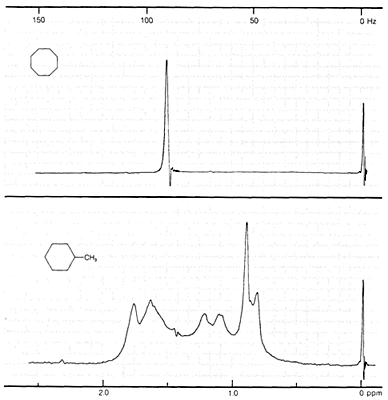
Some spectoscopic properties of cycloalkanes has been notable in the proton NMR spectra. The proton NMR spectra of alkanes and cycloalkanes are characteristic but difficult to interpret because the chemical shifts between the various kinds of protons are usually small. Although proton spectra of simple cycloalkanes, \(\ce{(CH_2)}_n\), show one sharp line at room temperature, when alkyl substituents are present, small differences in chemical shifts between the ring hydrogens occur and, with spin-spin splitting, provide more closely spaced lines than normally can be resolved. The complexity so introduced can be seen by comparing the proton spectra of cyclooctane and methylcyclohexane shown in Figure 12-1. For methyl-substituted cycloalkanes the methyl resonances generally stand out as high-field signals centered on \(0.9 \: \text{ppm}\), and the area of these signals relative to the other \(\ce{C-H}\) signals may be useful in indicating how many methyl groups there are (see Section 9-10K, especially Figure 9-46). However, in cyclopropanes the ring protons have abnormally small chemical shifts (\(\delta = 0.22\) for cyclopropane), which often overlap with the shifts of methyl groups \(\left( \delta \cong 0.9 \: \text{ppm} \right)\).
Although spectroscopic properties of cycloalkanes in proton spectra are not very useful for identification purposes, \(\ce{^{13}C}\) NMR spectra are very useful. Chain-branching and ring-substitution normally cause quite large chemical-shift changes, and it is not uncommon to observe \(\ce{^{13}C}\) shifts in cycloalkanes spanning \(35 \: \text{ppm}\). Some special features of application of \(\ce{^{13}C}\) NMR spectra to conformational analysis of cycloalkanes are described in Section 12-3D.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


