9.9: Raman Spectroscopy
- Page ID
- 22226
Raman spectroscopy often is a highly useful adjunct to infrared spectroscopy. The experimental arrangement for Raman spectra is quite simple in principle. Monochromatic light, such as from an argon-gas laser, is passed through a sample, and the light scattered at right angles to the incident beam is analyzed by an optical spectrometer.
Raman spectra arise as a result of light photons being “captured” momentarily by molecules in the sample and giving up (or gaining) small increments of energy through changes in the molecular vibrational and rotational energies before being emitted as scattered light. The changes in the vibrational and rotational energies result in changes in wavelength of the incident light. These changes are detected as lines falling both above and below the wavelength of the incident light. The line positions in Raman spectra always are reported in wave numbers. Highly efficient laser Raman spectrometers are commercially available.
Although changes in wavelength in Raman scattering correspond to absorption or emission of infrared radiation, infrared and Raman spectra are not always identical. Indeed, valuable information about molecular symmetry may be obtained by comparison of infrared and Raman spectra. When a bond is electrically symmetrical it does not absorb infrared radiation and, for this reason, symmetrical diatomic molecules such as \(H_2\) and \(O_2\), which are always electrically symmetrical, do not give infrared absorption spectra. However, excitation of symmetrical vibrations does occur in Raman scattering.\(^7\) In a molecule such as ethene, \(CH_2=CH_2\), the double-bond stretching vibration is symmetrical, because both ends of the molecule are the same. As a result, the double-bond stretching absorption is not observable in the infrared spectrum of ethene and is weak in all nearly symmetrically substituted ethenes. Nonetheless, this vibration appears strongly in the Raman spectrum of ethene and provides evidence for a symmetrical structure for ethene.
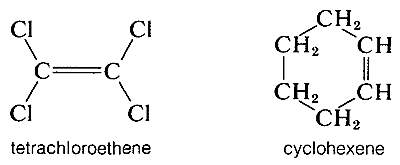
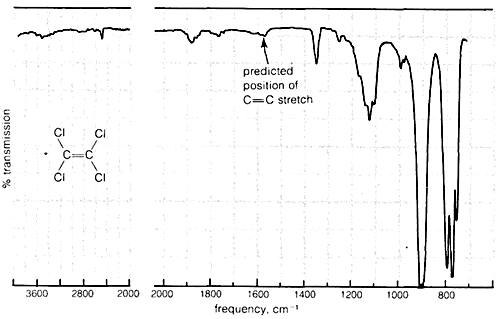
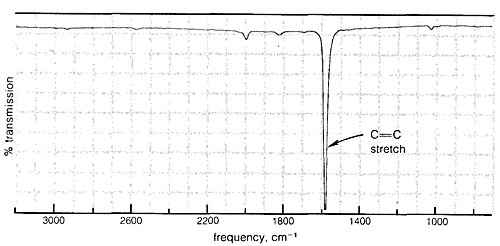
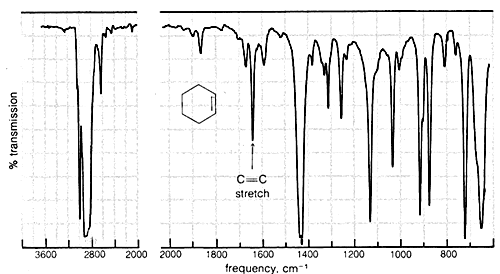
Absorption due to the stretching vibration of the double bond in tetrachloroethene (\(1570 \: \text{cm}^{-1}\) is strong in the Raman and absent in the infrared, whereas that arising from the less symmetrical double bond of cyclohexene (\(1658 \: \text{cm}^{-1}\)) is weak in the infrared and slightly stronger in the Raman.
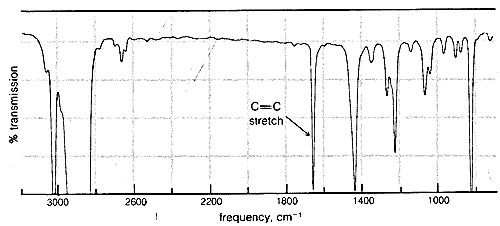
\(^7\)This is in accord with the spectroscopic "selection rules," derived from theoretical arguments, that predict which transitions between rotational and vibrational energy levels are "allowed" and which are "forbidden."
References
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


