8.10: The E1 Reaction
- Page ID
- 22219
Scope and Mechanism
Many secondary and tertiary halides undergo \(\text{E}1\) elimination in competition with the \(\text{S}_\text{N}1\) reaction in neutral or acidic solutions. For example, when tert-butyl chloride solvolyzes in \(80\%\) aqueous ethanol at \(25^\text{o}\), it gives \(83\%\) tert-butyl alcohol by substitution and \(17\%\) 2-methylpropene by elimination:
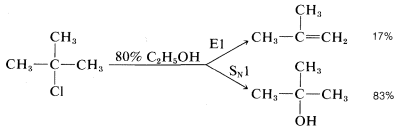
The ratio of substitution and elimination remains constant throughout the reaction, which means that each process has the kinetic order with respect to the concentration of tert-butyl halide. The \(\text{S}_\text{N}1\) and \(\text{E}1\) reactions have a common rate-determining step, namely, slow ionization of the halide. The solvent then has the choice of attacking the intermediate carbocation at the positive carbon to effect substitution, or at a \(\beta\) hydrogen to effect elimination:
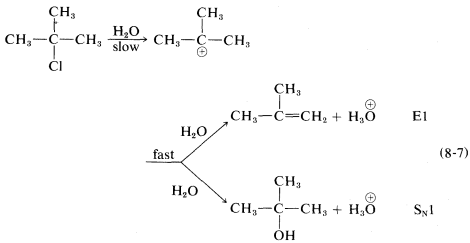
Factors influencing the \(\text{E}1\) reactions are expected to be similar to those for the \(\text{S}_\text{N}1\) reactions. An ionizing solvent is necessary, and for easy reaction the \(RX\) compound must have a good leaving group and form a relatively stable \(R^\oplus\) cation. Therefore the \(\text{E}1\) orders of reaction rates are \(X = I\) \(>\) \(Br\) \(>\) \(Cl\) \(>\) \(F\) and tertiary \(R\) \(>\) secondary \(R\) \(>\) primary \(R\).
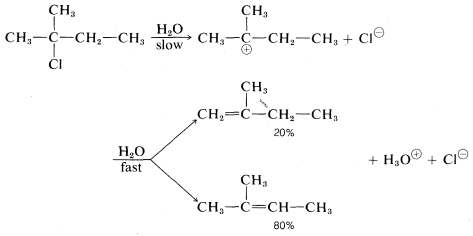
With halides such as 2-chloro-2-methylbutane, which can give different alkenes depending on the direction of elimination, the \(\text{E}1\) reaction is like the \(\text{E}2\) reaction in tending to favor the most stable or highly substituted alkene:
Rearrangement of Carbon Cations
Another feature of \(\text{E}1\) reactions (and also of \(\text{S}_\text{N}1\) reactions) is the tendency of the initially formed carbocation to rearrange, especially if a more stable carbocation is formed thereby. For example, the very slow \(\text{S}_\text{N}1\) solvolysis of neopentyl iodide in methanoic acid leads predominantly to 2-methyl-2-butene:
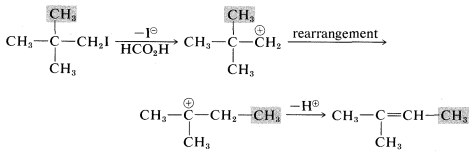
In this reaction, ionization results in migration of a methyl group with its bonding pair of electrons from the \(\beta\) to the \(\alpha\) carbon, thereby transforming an unstable primary carbocation to a relatively stable tertiary carbocation. Elimination of a proton completes the reaction.
Rearrangements involving shifts of hydrogen (as \(H:^\ominus\)) occur with comparable ease if a more stable carbocation can be formed thereby:
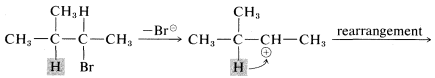
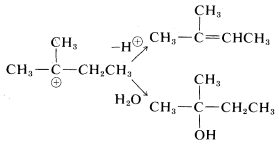
Rearrangements of carbocations are among the fastest organic reactions known and must be reckoned with as a possibility whenever carbocation intermediates are involved.
Acid-Catalyzed Elimination Reactions
Alcohols and ethers rarely undergo substitution or elimination unless strong acid is present. As we noted in Section 8-7D the acid is necessary to convert a relatively poor leaving group (\(HO^\ominus\), \(CH_3O^\ominus\)) into a relatively good one (\(H_2O\), \(CH_3OH\)). Thus the dehydration of alcohols to alkenes is an acid-catalyzed reaction requiring strong acids such as sulfuric or phosphoric acid:
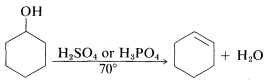
These are synthetically useful reactions for the preparation of alkenes when the alkene is less available than the alcohol. They can occur by either the \(\text{E}1\) or \(\text{E}2\) mechanism depending on the alcohol, the acid catalyst, the solvent, and the temperature.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


