7.6: Carboxylic Acids
- Page ID
- 22193
By the IUPAC system, the suffix -oic is added to the prefix identifying the hydrocarbon chain that includes the carboxyl carbon:
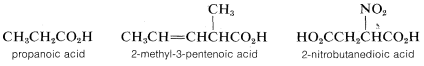
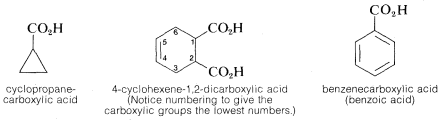
Situations arise when it is necessary to consider the parent as a one-carbon chain. In such circumstances, \(RCO_2H\) becomes a substituted carboxylic acid. This variation is met most frequently when \(R\) is a cycloalkyl or aryl group:
The substituent name for \(-CO_2H\) is carboxy:
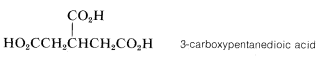
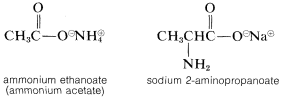
For salts of carboxylic acids, the -oic suffix of the acid becomes -oate with the counter ion named as a separate word:
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


