7.11: Single- or Multiple-Word Names
- Page ID
- 22197
A troublesome point in naming chemical compounds concerns the rules governing when a compound is to be written as a single word (as methylamine) or as two or more words (as methyl chloride). To solve this problem, you must determine whether the principle or parent function is an element or a compounds in its own right; if it is either one, then the name is written as a single word.
The following examples should help to clarify the system. In each name, the part of the name that denotes the parent compound\(^2\) is italicized:
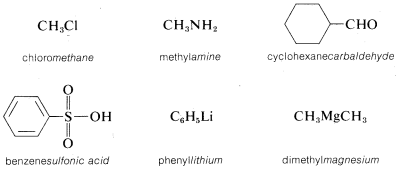
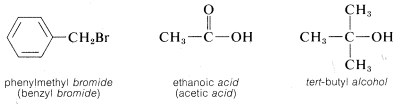
However, if the parent function cannot be constructed as being a real compound, the name is correctly written as two or more words. For example, \(CH_3Cl\) could be named as a chloride, in which case we use two words, methyl chloride, to describe it. A chloride, or any halide, is a class of compound, not a specific compound. To identify a specific halide, the adjective that describes the halide is written as a separate word preceding the class name. Examples follow in which the class name is italicized.\(^3\)
\(^2\)The parent compounds designated here as amine, carbaldehyde, and sulfonic acid are properly ammonia, methanal, and sulfurous acid (\(HSO_3H\)) when no substituent groups are attached.
\(^3\)These word-separated names sometimes are called radicofunctional names.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


