3.1: Prelude to Organic Nomenclature
- Page ID
- 79302
Organic chemists, regardless of what languages they speak, can communicate with one another about their chemical work simply by writing equations and structural formulas. But this is a slow process if the molecules are complicated, and is not well suited for conversation (try describing a structural formula of a complex molecule to someone). For more rapid and efficient communication we need to have names for compounds, and we should have every reason to hope that, after 100 years, the names now in use would be clear, unambiguous, easy to pronounce, easy to spell and to remember, as well as being amenable to arrangement in alphabetical order. But, even more, we should hope that the names of organic compounds would contain enough information so we could generate the proper structures from them, and conversely, if we know the structures then the system would have simple enough rules that we could construct universally recognized and accepted names.
Unfortunately, these splendid ideals have not yet been realized. A good part of the problem is that people are resistant to change and especially resistant to changes in names. To give an example, the carboxylic acid, \(\ce{CH_3-CO_2H}\), commonly is known as "acetic acid". The name arises from the Latin word acetum, for sour wine or vinegar, and acetic acid is the principal constituent, besides, water, of vinegar. A similarly common compound is called "acetone" and, in the ideal world, acetone should be structurally related to acetic acid. But acetone is \(\ce{CH_3-CO-CH_3}\), and the name arises only because acetone is formed by strong heating of the calcium salt of acetic acid, \(\ce{(CH_3-CO_2)_2Ca} \rightarrow \ce{CH_3-CO-CH_3} + \ce{CaCO_3}\), a reaction that is of no current importance whatsoever. Better nomenclature systems use names based on the name of the hydrocarbon with the same number of carbons in the longest continuous chain in the molecule. On this basis, \(\ce{CH_3CO_2H}\) is related to ethane and is called ethanoic acid, whereas \(\ce{CH_3-CO-CH_3}\) with three carbons is related to propane and called 2-propanone.
As far as possible, we shall use these names as our first choices, because organic chemistry is growing too fast to sustain the present chaos of nonsystematic nomenclatures in current use. You might well ask why nonsystematic names persist for so long. The reasons are complex and variable. Alchemists intentionally used abstruse names and symbolism to disguise what they really were working with. Chemical industry, especially in the drug area, has practiced much the same thing in using unintelligible trade names and codes for proprietary products. Obviously, everyone who handles or sells chemicals is not a chemist, and to the nonchemist, a short nonsystematic name will make more sense than a longer systematic name. A salesman who markets tons of "acrolein", \(\ce{CH_2=CH-CH=O}\), has little reason to adopt the systematic name, 2-propenal.
People probably persist in using nicknames for chemicals for much the same reason that they use nicknames for people. Nicknames are less formal, usually shorter, and imply familiarity with the subject. Another cogent reason to resist dramatic changes in chemical nomenclature is that it would make the current and earlier literature archaic or even unintelligible. Universal adoption tomorrow of a nomenclature system different from the one we use here would render this book instantly obsolete. As a result, changes usually are made in small steps and may not be really effective until a generation or more passes. (Consider in this context the efforts to convert monetary systems and weights and measures to the decimal system.)
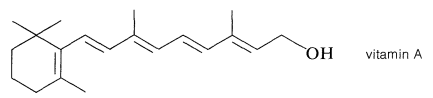
Ideally, every organic substance should have a completely descriptive, systematic name to permit only one structural formula to be written for it. This ideal has been approached closely in some of the current nomenclature systems but, unfortunately, truly systematic nomenclature for very complicated compounds is often hopeless for conversational or routine scriptorial purposes. As a result, we will at times resort to using (common) trivial names, especially if it is impractical to do otherwise. Clearly, the description 9-(2,6,6-trimethyl-1-cyclohexenyl)-3,7-dimethyl-2,4,6,8-nonatetraen-1-ol has phonetic disadvantages as a handy name for vitamin A:
A very important consideration for becoming more familiar with the systematic names is their increasing use in indexing systems. When organic chemists dealt with relatively few compounds, it was possible to accommodate a wide variety of special nomenclature customs. However, the rapid growth of knowledge in the past twenty years, which probably has doubled the number of organic compounds, has also enormously increased the burden on those who dedicate themselves to making this knowledge easily available to others by indexing the current literature. A natural reaction is to discard common names in favor of more systematic ones and to develop numerical designations suitable for computer processing. The difference in sizes of the Chemical Abstracts\(^1\) indexes for the years 1907-1916 and for the current year should be convincing as to the need for having systematic names become more widely used and important. But the fact remains that the naming systems used in indexing are not always the same as those used in practice, and we are left with the necessity of having to know both.
Learning the nomenclature of organic compounds has many of the elements of learning a language, be it Latin or Fortran. Fortunately, like a language it does not have to be learned all at once. One can become familiar with naming of simple hydrocarbons, then study their chemistry (avoiding that part which involves compounds with as yet unlearned names), proceed to the naming of alkenes, study their chemistry, and so on. This is a very simple and natural way but can be inconvenient in a textbook if one wants to review the nomenclature of more than one class of compounds at a time.
In this chapter, we consolidate the nomenclature of a number of classes of compounds - an undertaking that may not seem very logical to someone who will soon be troubled enough with the chemistry of these compounds let alone their names. We recommend, however, a thorough study now of alkane and haloalkane nomenclature (Section 3-1) followed by a more cursory examination of the rest of the chapter. Then, as unfamiliar names arise, you can quickly review the basic rules for alkanes and proceed to the new class you have encountered. The idea is to have many of the important rules in one place. Nomenclature rules for other types of compounds are given in Chapter 7.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


