3.3: Cycloalkanes
- Page ID
- 22166
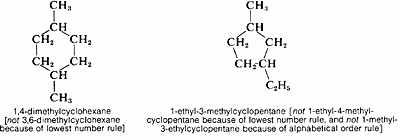
The cycloalkanes with one ring have the general formula \(C_nH_{2n}\), and are named by adding the prefix cyclo- to the name of the corresponding continuous-chain alkane having the same number of carbon atoms as the ring. Substituents are assigned numbers consistent with their position in such a way as to give the lowest numbers possible for the substituent positions:
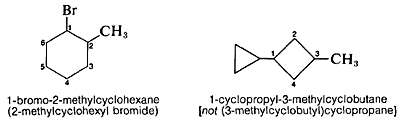
The substituent groups derived from cycloalkanes by removing one hydrogen are named by replacing the ending -ane of the hydrocarbon with -yl to give cycloalkyl. Thus cyclohexane becomes cyclohexyl, cyclopentane becomes cyclopentyl, and so on. Remember, the numbering of the cycloalkyl substituent starts at the position of attachment, and larger rings take precedence over smaller rings:
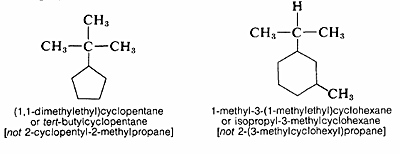
When a cycloalkane has an alkyl substituent, the compound could be called either an alkylcycloalkane or a cycloalkylalkane. The alkylcycloalkane name is the proper one in naming alkyl substituted cycloalkanes.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


