V. Summary
- Page ID
- 24617
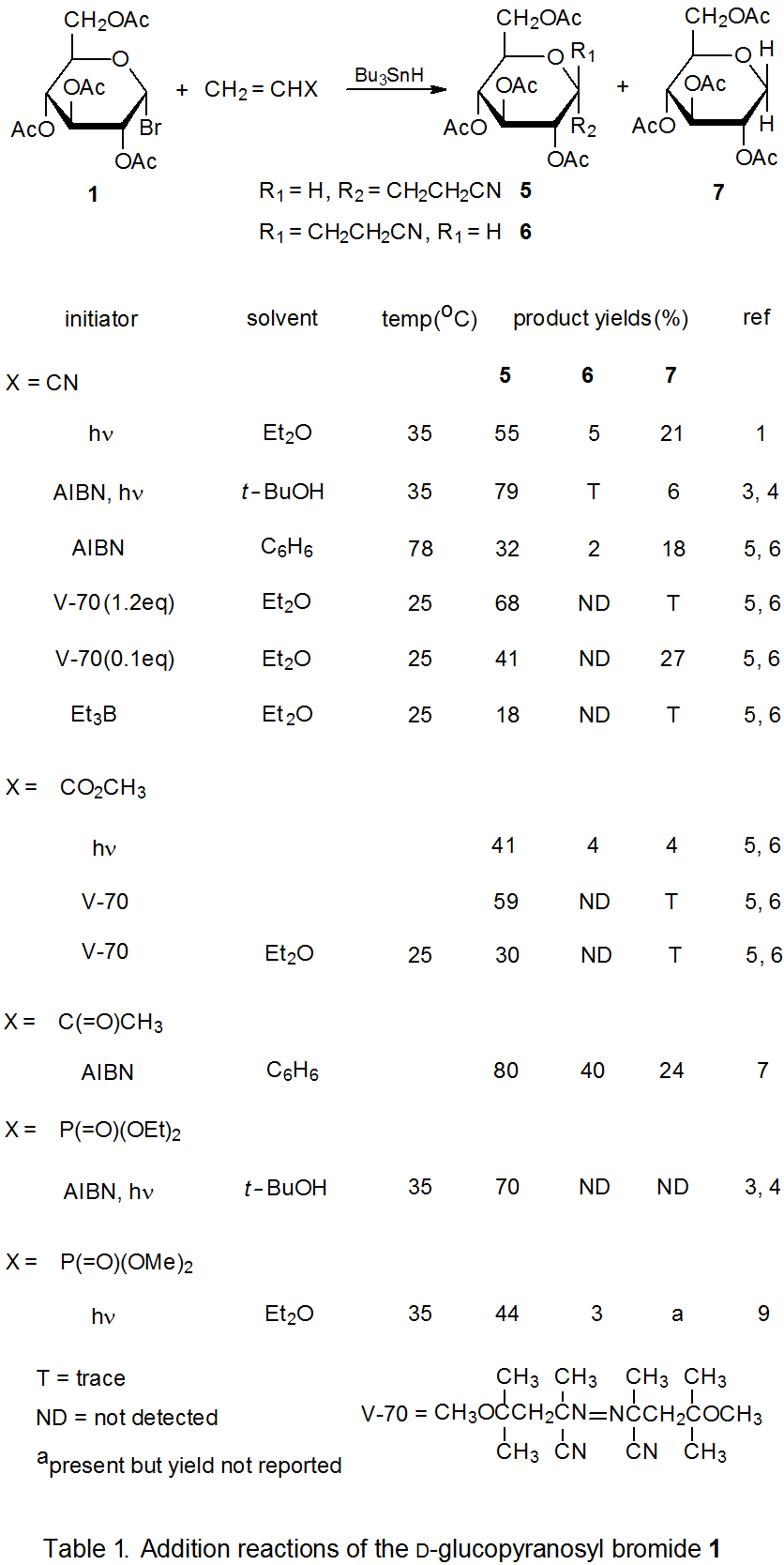
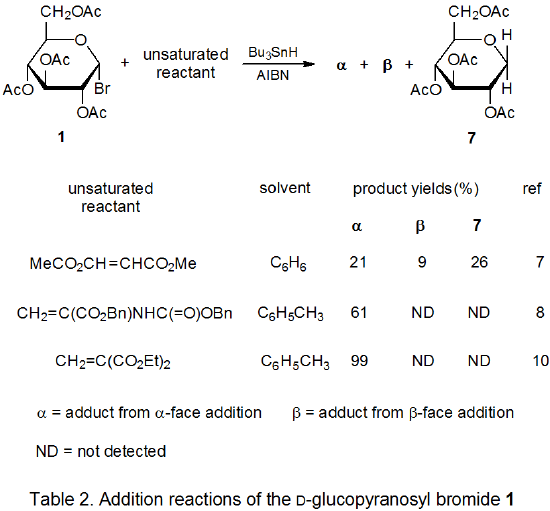
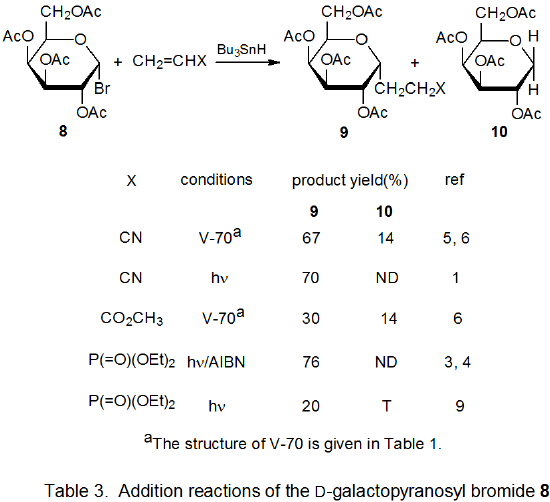
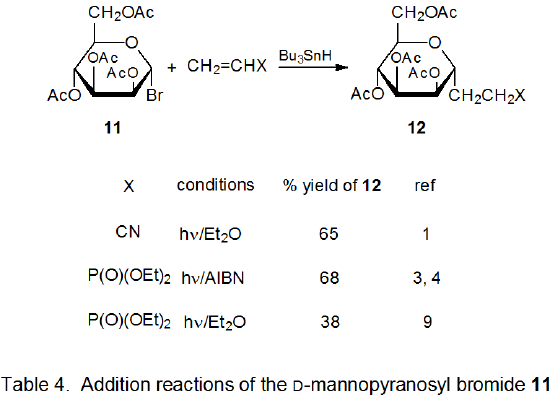
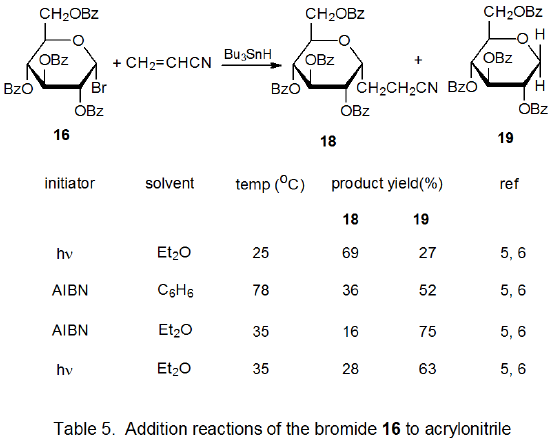
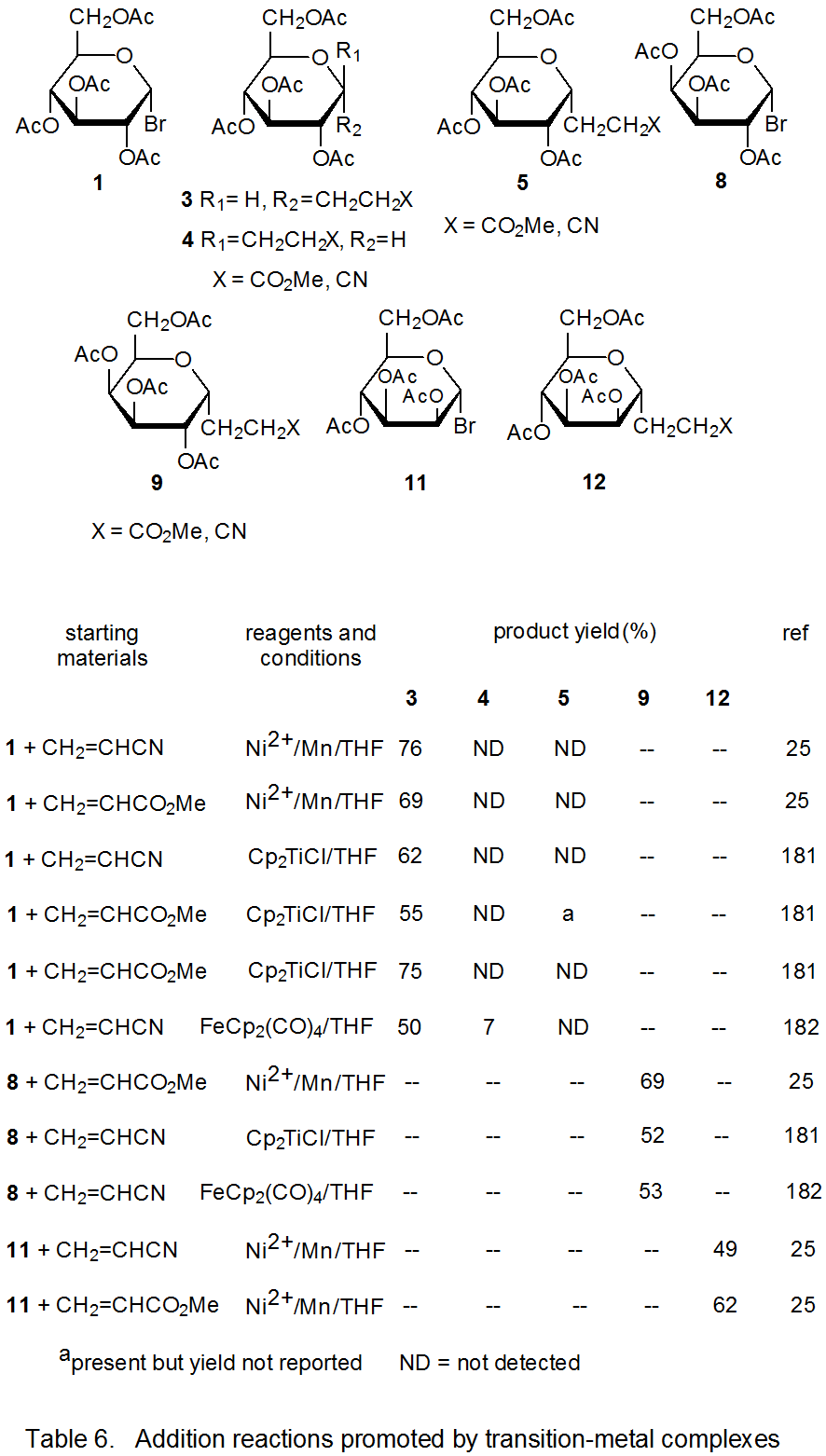
Radical addition to compounds with carbon–carbon multiple bonds can take place by several reaction mechanisms. The most common of these is a chain reaction that is characterized by a bimolecular, chain-transfer step. This type of reaction governs addition to unsaturated compounds of radicals centered on various carbon atoms in pyranoid and furanoid rings. These reactions are regiospecific and often highly stereoselective. Since most carbon-centered radicals, including those derived from carbohydrates, are nucleophilic, successful addition requires an electron-withdrawing substituent attached to one of the carbon atoms of the multiple bond. The primary limitation placed on this type of addition reaction is that it is in competition with a hydrogen-atom abstraction reaction that causes simple reduction of the carbohydrate derivative.
Addition reactions also can occur by unimolecular chain-transfer. This type of reaction also takes place with radicals centered on various carbon atoms in pyranoid and furanoid rings. Most of these reactions involve addition of a carbohydrate radical to an allylic or vinylic stannane followed by loss of a tin-containing substituent. An advantage to this type of reaction is that competition with simple reduction can be largely avoided. A disadvantage is that 1,3-tin migration can occur in allylic stannanes; such migration places a synthetic limitation on the usefulness of the reaction.
Although the unsaturated compound in a typical addition reaction is a noncarbohydrate, addition also occurs to unsaturated carbohydrates. The carbon–carbon double bond in such reactions usually is part of an enol ether or an α,β-unsaturated ketone or lactone and the product is often a C-disaccharide.
Most addition reactions to carbon–carbon triple bonds involve a tri-n-butyltin radical adding to a triple bond in a carbohydrate. The structures of the carbohydrates are such that the vinylic radicals produced often undergo a cyclization reaction.