11.2: Carboxylic Acid Derivatives
- Page ID
- 106358
The functional groups at the heart of this chapter are called carboxylic acid derivatives: they include carboxylic acids themselves, carboxylates (deprotonated carboxylic acids), amides, esters, thioesters, and acyl phosphates.

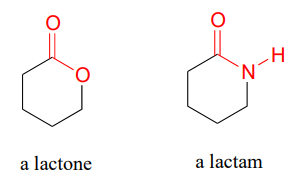
Cyclic esters and amides are referred to as lactones and lactams, respectively.
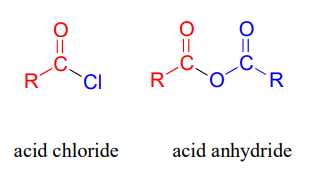
Carboxylic acid anyhydrides and acid chlorides, which also fall under the carboxylic acid derivative category, are not generally found in biomolecules but are useful intermediates in laboratory synthesis. They are discussed in a section on laboratory reactions at the end of this chapter.
Carboxylic acid derivatives can be distinguished from aldehydes and ketones by the presence of a group containing an electronegative heteroatom - usually oxygen, nitrogen, or sulfur – bonded directly to the carbonyl carbon. You can think of a carboxylic acid derivative as having two sides. One side is the acyl group, which is the carbonyl plus the attached alkyl (R) group. In the specific cases where R is a hydrogen or methyl, chemists use the terms formyl and acetyl group, respectively. One the other side is the heteroatom-linked group: in this text, we will sometimes refer to this component as the ‘acyl X' group (this, however, is not a standard term in organic chemistry).
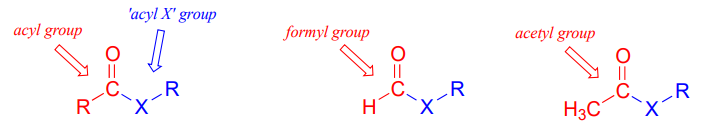
Notice that the acyl X groups are simply deprotonated forms of other functional groups linked to the acyl group: in an amide, for example, the acyl X group is an amine, while in an ester the acyl X group is an alcohol.
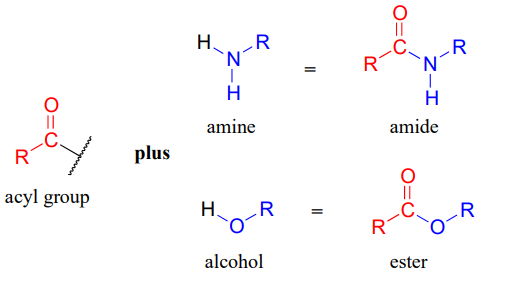
What is the ‘acyl X’ group in:
- an acid anhydride?
- a thioester?
- a carboxylic acid?
- an acyl phosphate?
Draw the structures indicated:
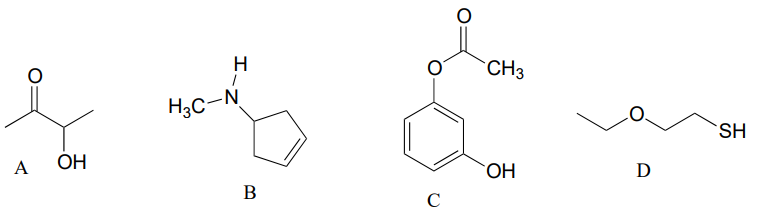
- compound A after is has been acetylated (ie. an acetyl group added)
- compound B after it has been formylated
- compound C after it has been formylated
- Compound D after it has been acetylated
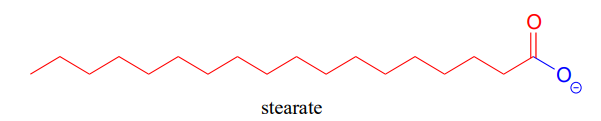
'Fatty acid' molecules such as stearate are carboxylates with long carbon chains for acyl groups.
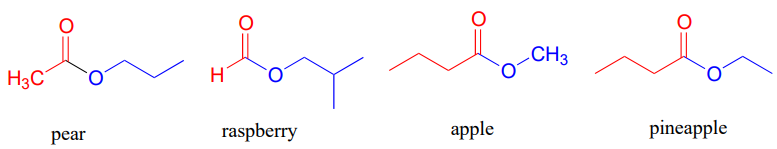
The aromas of many fruits come from small ester-containing molecules:
The 'peptide bonds' that link amino acids together in proteins are amides.
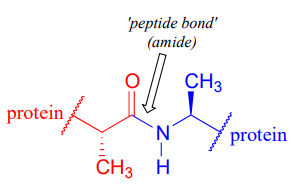
Acetyl-Coenzyme A, a very important two carbon (acetyl group) 'building block' molecule in metabolism, is characterized by reactions at its thioester functional group:
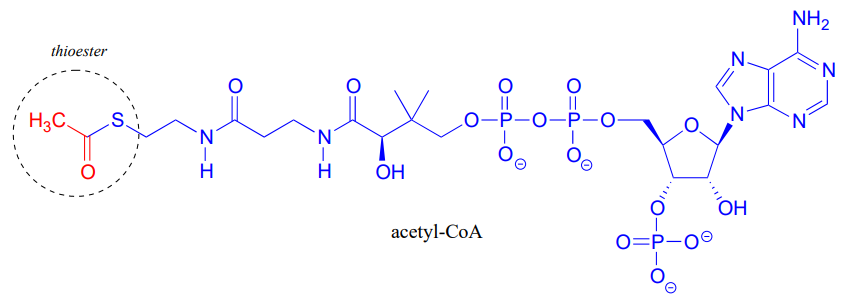
There are two amide groups in acetyl-CoA: identify them.
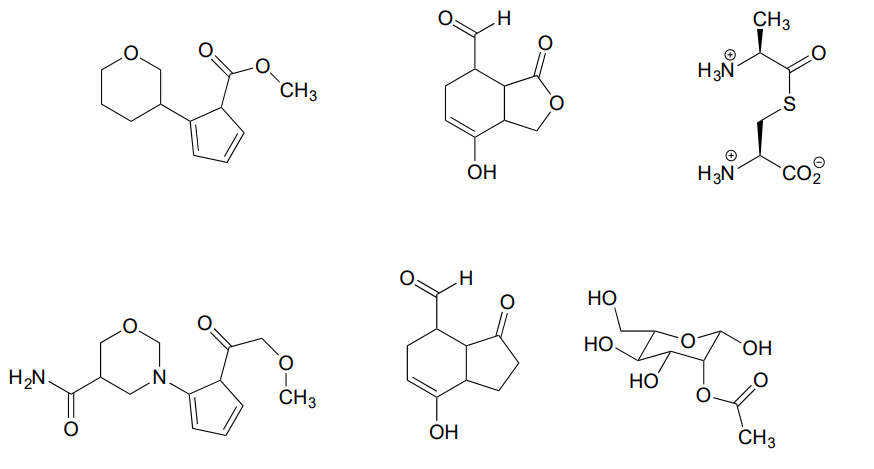
Name all carboxylic acid derivative groups in the molecules below.