8.5: Leaving Groups
- Page ID
- 106340
Next, we investigate what makes a good leaving group. It's really quite straightforward: everything that we learned in chapter 7 about evaluating base strength will apply to leaving groups:
Weaker bases are better leaving groups
In our general discussion of nucleophilic substitution reactions, we have until now been using chloride ion as our common leaving group. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. Iodide, which is the least basic of the four common halides (\(F\), \(Cl\), \(Br\), and \(I\)), is the best leaving group among them. Fluoride is the least effective leaving group among the halides, because fluoride anion is the most basic. This rule applies to both \(S_N2\) and \(S_N1\) reactions, because in both cases the rate-determining step involves loss of the leaving group.
best leaving group \(I\)- > \(Br\)- > \(Cl\)- > \(F\)- worst leaving group
This trend is evident when you compare the relative rates of \(S_N2\) reactions of four halomethanes with a common nucleophile and solvent: iodomethane reacts fastest, fluoromethane the slowest.
fastest \(S_N2\) reaction \(CH_3I\) > \(CH_3Br\) > \(CH_3Cl\) > \(CH_3F\) slowest \(SN_2\) reaction
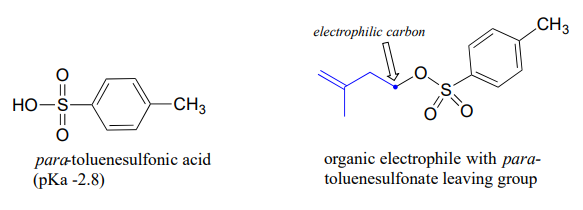
The conjugate base of toluenesulfonic acid is a leaving group commonly used in the organic synthesis laboratory. Toluenesulfonic acid is a strong organic acid with a \(pK_a\) of -2.8, so its conjugate base is a weak base and excellent leaving group.
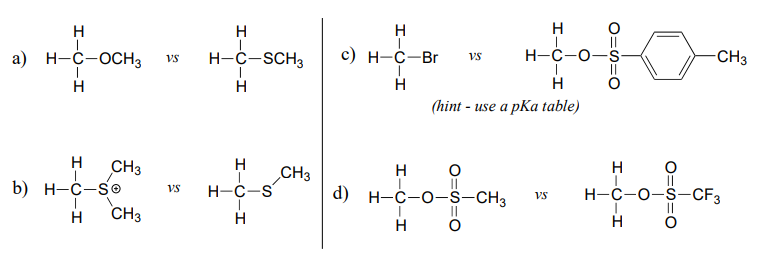
In each pair (A and B) below, which electrophile would be expected to react more rapidly with cyanide ion nucleophile in acetone solvent? Explain your reasoning.
Beginning later in this chapter and throughout the rest of our study of organic reactivity, we will see examples of leaving group 'activation': in other words, conversion of a strong base/poor leaving group into a weak base/good leaving group. In some cases this is as simple as protonation: an acidic group may be positioned in the active site in order to protonate a poor leaving group (eg. hydroxide ion in the case of an alcohol) as it leaves, thus converting it into a weak base and good leaving group. In many other enzymatic reactions, alcohols are converted into phosphates, which can be excellent biochemical leaving groups. We will learn much more about the structure and reactions of organic phosphate compounds in chapter 9.


