5.6A: Overview of Rotary Evaporation
- Page ID
- 95729
It is very common for a desired compound to be dissolved in a solvent during regular manipulations in the laboratory. Solvents are used in separatory funnel extractions and column chromatography, and the solvent must be removed in order to isolate te desired compound. Solvents are regularly chosen that have lower boiling points than the compound of interest, so that there is some mechanism for their removal. In theory, a solution could simply be placed on a heat source to boil away the lower-boiling solvent, but this approach is not often used.
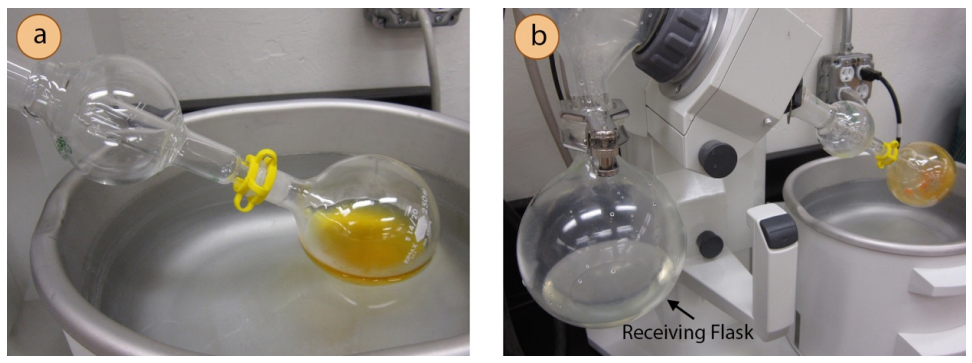
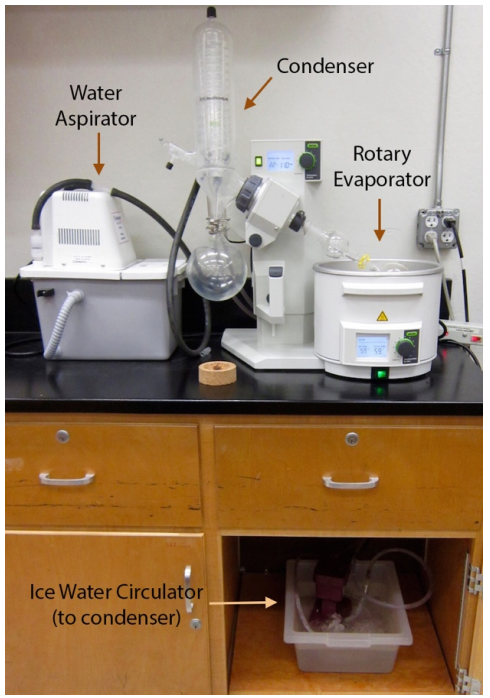
The preferred method for solvent removal in the laboratory is by use of a rotary evaporator (Figure 5.65), also known as a "rotovap". A rotary evaporator is essentially a reduced pressure distillation: a solution in a round bottomed flask is placed in the water bath of the apparatus (Figure 5.66a), and rotated while the system is partially evacuated (by a water aspirator or vacuum pump). The reduced pressure in the apparatus causes the solvent to boil at a lower temperature than normal (see vacuum distillation), and rotating the flask increases the liquid's surface area and thus the rate of evaporation. The solvent vapor condenses when it comes into contact with a water condenser (Figure 5.65) and drips into a receiving flask (Figure 5.66b). When the solvent is removed, the concentrated compound is left in the flask. One difference between distillation and rotary evaporation is that the distillate is most often retained in distillation while the residue is retained in rotary evaporation.
Removal of solvent by a rotary evaporator is superior to evaporation under atmospheric pressure for many reasons. The process is much quicker (often takes less than 5 minutes), uses lower temperatures (so decomposition is unlikely), and uses less energy than boiling with a heat source. Since low pressure is used, a rotary evaporator is also quite efficient at removing the last traces of residual solvent from a solution.