29.9: Naturally Occurring Polymers
- Page ID
- 80414
There are a number of naturally occurring polymeric substances that have a high degree of technical importance. Some of these, such as natural rubber (Section 13-4), cellulose, and starch (Section 20-7), have regular structures and can be regarded as being made up of single monomer units. Others, such as wool, silk (Section 25-8A), and deoxyribonucleic acid (Section 25-13A) are copolymers. Because we already have considered the chemistry of most of these substances, we shall confine our attention here to wool and collagen, which have properties related to topics discussed previously in this chapter.
Wool
The structure of wool is more complicated than that of silk fibroin (Figure 25-13) because wool, like insulin (Figure 25-8) and lysozyme (Figure 25-15), contains a considerable quantity of cystine, which provides \(\ce{-S-S}-\) (disulfide) cross-links between the peptide chains. These disulfide linkages play an important part in determining the mechanical properties of wool fibers because if the disulfide linkages are reduced, as with ammonium mercaptoethanoate solution, the fibers become much more pliable.
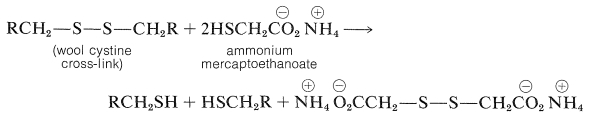
Advantage is taken of this reaction in the curling of hair, the reduction and curling being followed by restoration of the disulfide linkages through treatment with a mild oxidizing agent.
Collagen
The principal protein of skin and connective tissue is called collagen and is primarily constituted of glycine, proline, and hydroxyproline. Collagen is made up of tropocollagen, a substance with very long and thin molecules (\(14 \times 2900 \: \text{Å}\), MW about 300,000). Each tropocollagen molecule consists of three twisted polypeptide strands. When collagen is boiled with water, the strands come apart and the product is ordinary cooking gelatin. Connective tissue and skin are made up of fibrils, \(200 \: \text{Å}\) to \(1000 \: \text{Å}\) wide, which are indicated by x-ray diffraction photographs to be composed of tropocollagen molecules running parallel to the long axis. Electron micrographs show regular bands, \(640 \: \text{Å}\) apart, across the fibrils, and it is believed that these correspond to tropocollagen molecules, all heading in the same direction but regularly staggered by about a fourth of their length (Figure 29-10).
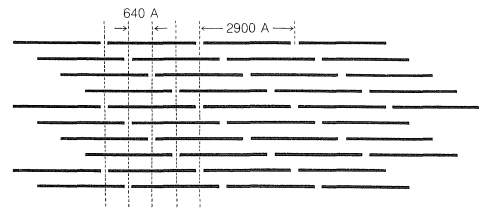
The conversion of collagen fibrils to leather presumably involves formation of cross-links between the tropocollagen molecules. Various substances can be used for the purpose, but chromium salts act particularly rapidly.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


