29.3: Types of Polymers
- Page ID
- 22394
Polymers can be classified in several different ways - according to their structures, the types of reactions by which they are prepared, their physical properties, or their technological uses.
From the standpoint of general physical properties, we usually recognize three types of solid polymers: elastomers, thermoplastic polymers, and thermosetting polymers. Elastomers are rubbers or rubberlike elastic materials. Thermoplastic polymers are hard at room temperature, but on heating become soft and more or less fluid and can be molded. Thermosetting polymers can be molded at room temperature or above, but when heated more strongly become hard and infusible. These categories overlap considerably but are nonetheless helpful in defining general areas of utility and types of structures.
The structural characteristics that are most important to determining the properties of polymers are:
- the degree of rigidity of the polymer molecules,
- the electrostatic and van der Waals attractive forces between the chains,
- the degree to which the chains tend to form crystalline domains, and
- the degree of cross-linking between the chains.
Of these, cross-linking is perhaps the simplest and will be discussed next.
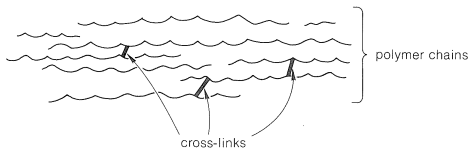
Consider a polymer made of a tangle of molecules with long linear chains of atoms. If the intermolecular forces between the chains are small and the material is subjected to pressure, the molecules will tend to move past one another in what is called plastic flow. Such a polymer usually is soluble in solvents that will dissolve short-chain molecules with chemical structures similar to those of the polymer. If the intermolecular forces between the chains are sufficiently strong to prevent motion of the molecules past one another the polymer will be solid at room temperature, but will usually lose strength and undergo plastic flow when heated. Such a polymer is thermoplastic. A cross-link is a chemical bond between polymer chains other than at the ends. Cross-links are extremely important in determining physical properties because they increase the molecular weight and limit the translational motions of the chains with respect to one another. Only two cross-links per polymer chain are required to connect all the polymer molecules in a given sample to produce one gigantic molecule. Only a few cross-links (Figure 29-1) reduce greatly the solubility of a polymer and tend to produce what is called a gel polymer, which, although insoluble, usually will absorb (be swelled by) solvents in which the uncross-linked polymer is soluble. The tendency to absorb solvents decreases as the degree of cross-linking is increased because the chains cannot move enough to allow the solvent molecules to penetrate between the chains.
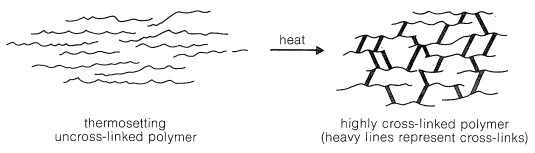
Thermosetting polymers normally are made from relatively low-molecular-weight, usually semifluid substances, which when heated in a mold become highly cross-linked, thereby forming hard, infusible, and insoluble products having a three-dimensional network of bonds interconnecting the polymer chains (Figure 29-2).
Polymers usually are prepared by two different types of polymerization reactions - addition and condensation. In addition polymerization all of the atoms of the monomer molecules become part of the polymer; in condensation polymerization some of the atoms of the monomer are split off in the reaction as water, alcohol, ammonia, or carbon dioxide, and so on. Some polymers can be formed either by addition or condensation reactions. An example is polyethylene glycol, which, in principle, can form either by dehydration of 1,2-ethanediol (ethylene glycol), which is condensation, or by addition polymerization of oxacyclopropane (ethylene oxide):\(^1\)
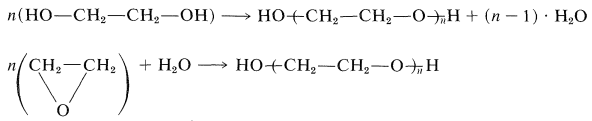
Other addition polymerizations were discussed previously, including poly-1,3-cyclopentadiene, alkene polymers (Section 19-8), polyalkadienes (Section 13-4), polyfluoroalkenes (Section 14-7D), and polymethanal (Section 16-4B).
\(^1\)Regardless of whether the same polymer would be obtained by polymerization starting with different monomers, the products usually are named to correspond to the starting material. Thus polyethylene glycol and polyethylene oxide would not be used interchangeably for \(\ce{HO-(CH_2CH_2-O)}_n \ce{-H}\).
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


