18.8: Reactions at the Carbonyl Carbon of Acid Derivatives
- Page ID
- 22293
Displacement Reactions
Hydrolysis of most acid derivatives to the parent acids is acid- or base-catalyzed:
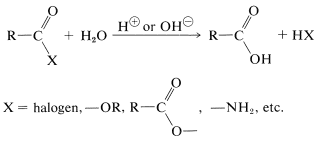
However, acyl halides and anhydrides usually hydrolyze rapidly without the aid of an acidic or basic catalyst, when in solution. It is important to recognize that an insoluble acyl halide or anhydride often reacts slowly with water. Esters and amides hydrolyze much more slowly, and for useful rates require a catalyst. The hydrolysis of amides is of exceptional importance in biochemistry and will be discussed in more detail in Chapters 24 and 25.
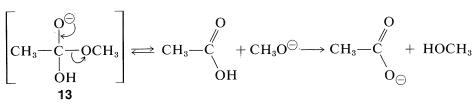
Acid-catalyzed hydrolysis of esters is the reverse of acid-catalyzed ester formation discussed previously. Base-induced ester hydrolysis (saponification) is an irreversible reaction. The initial step is the attack of hydroxide ion at the carbonyl carbon:

The intermediate anion, \(13\), so formed then either loses \(\ce{OH}^\ominus\) and reverts to the original ester, or it loses \(\ce{CH_3O}^\ominus\) to form the acid. The overall reaction is irreversible because once the acid is formed, it immediately is converted to the carboxylate anion, which is stabilized to such a degree that it is not attacked by the alcohol and will not reform the starting ester. Consequently, the reaction goes to completion in the direction of hydrolysis:
Ester interchange is closely related to ester hydrolysis. This is a base-catalyzed reaction that is useful to replace the alcohol group of an ester with a different alcohol group. The catalyst is alkoxide ion and the equilibrium constant is close to unity, unless the alcohols differ greatly in size. An example is
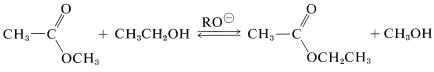
in which \(\ce{RO}^\ominus\) is either \(\ce{CH_3O}^\ominus\) or \(\ce{CH_3CH_2O}^\ominus\).
The formation of esters from acid chlorides and anhydrides according to the following equation has been discussed:
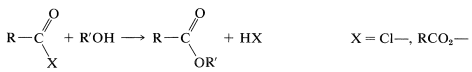
Amides can be obtained from acyl halides, carboxylic anhydrides, or esters with amines or ammonia. The mechanisms of these reactions are very similar to the corresponding reactions of alcohols:
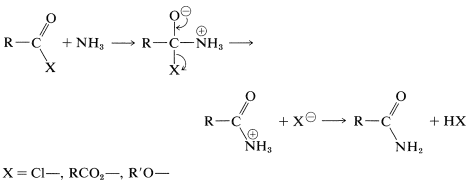
We will discuss this kind of reaction further in Chapters 24 and 25.
Reactions with Organometallic Compounds
The reactions of several carboxylic acid derivatives with organomagnesium and organolithium compounds were described in Section 14-12. The key step in these reactions is addition of the organometallic compound, as \(\ce{R}^{\delta \ominus} \ce{M}^{\delta \oplus}\), to the carbonyl group. For a Grignard reagent,

The reaction normally does not stop at this stage; \(\ce{MgXZ}\) is eliminated and the resulting ketone rapidly reacts with another molecule of organometallic compound. On hydrolysis, a tertiary alcohol is formed with at least two identical alkyl groups on the tertiary carbon:
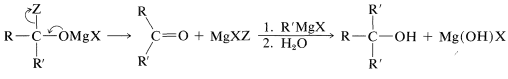
Reduction of Acid Derivatives
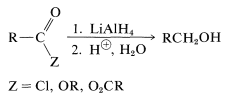
Esters, chlorides, and anhydrides are reduced by lithium aluminum hydride in the same general way as the parent acids (Section 18-3C), the difference being that no hydrogen is evolved. The products after hydrolysis are primary alcohols:
Nitriles can be reduced to amines by lithium aluminum hydride. An imine salt is an intermediate product; if the reaction is carried out under the proper conditions, this salt is the major product and provides an aldehyde on hydrolysis (see Section 16-4C):
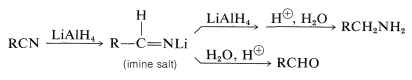
Amides are reduced to primary amines, and \(\ce{N}\)-substituted amides to secondary and tertiary amines:
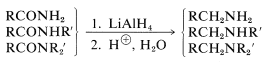
Borane also will reduce esters, amides, and nitriles to the same products as does \(\ce{LiAlH_4}\), but with reduced reactivity (Table 16-6).
Although lithium aluminum hydride and boranes are very useful reagents, they are expensive and impractical to employ on a large scale. Other methods of reduction then may be necessary. Of these, the most important are reduction of esters with sodium and ethanol (acids do not react readily),
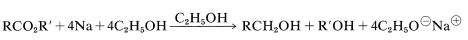
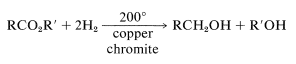
and high-pressure hydrogenation over a copper chromite catalyst,
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


