10.10: Alkylation of Alkenes
- Page ID
- 22246
Addition of a saturated hydrocarbon \(\left( \ce{R-H} \right)\) to an alkene to yield a saturated hydrocarbon of higher molecular weight is known as alkylation:

Such reactions are used by the petroleum industry to produce medium-molecular-weight hydrocarbons from smaller molecules. A particularly important example is afforded by the addition of 2-methylpropane to 2-methylpropene in the presence of sulfuric acid or anhydrous hydrogen fluoride to yield 2,2,4-trimethylpentane:
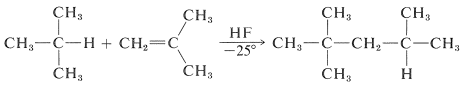
The overall reaction appears to be different from any so far discussed, because it involves addition of a nonpolar reagent \(\left( \ce{RH} \right)\) to an alkene bond.
The key to the mechanism of hydrocarbon alkylation was provided by the discovery by P. D. Bartlett, in 1940, that a carbocation can react rapidly with a hydrocarbon having a tertiary hydrogen to yield a new carbocation and a new hydrocarbon. Some of these "hydrogen-transfer" reactions are extraordinarily fast and may be complete in seconds or less. The hydrogen is transferred with both bonding electrons \(\left( \ce{H}^\ominus \right)\). For example,

With the knowledge that the hydrogen transfer is fast, the alkylation of 2-methylpropene with 2-methylpropane can be formulated as involving first polymerization of two 2-methylpropene molecules under the influence of the sulfuric acid catalyst to give the same octyl cation as was postulated for the dimerization of 2-methylpropene:
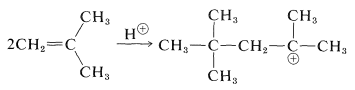
The octyl cation then can undergo a hydrogen-transfer reaction with 2-methylpropane to furnish 2,2,4-trimethylpentane and a tert-butyl cation:
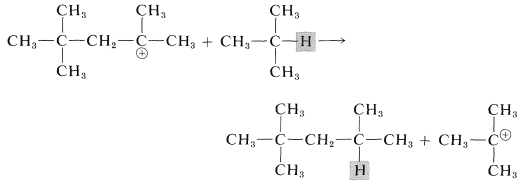
Attack by the tert-butyl cation on another molecule of 2-methylpropene produces an eight-carbon tertiary cation, which them proceeds to another molecule of "alkylate":
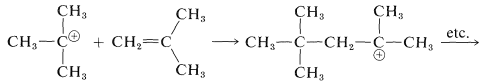
This is an important example of a cationic chain reaction.
References
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


