28.5: Color and Constitution
- Page ID
- 22385
Visible light is electromagnetic radiation having a rather narrow range of wavelengths (\(400\)-\(800 \: \text{nm}\)). A black substance absorbs all wavelengths of visible light. Selective absorption of visible light by a substance imparts color, but the color is not that of the light absorbed but instead of the residual light that the substance transmits or reflects. For example, a compound that absorbs in the region \(435\)-\(480 \: \text{nm}\) removes blue light from the visible spectrum, and the residual light is recognized by the eye as being yellow. The relationship of the observed color to wavelength of light absorbed is shown in Table 28-1. It is customary to call the color observed the complementary color or the subtraction color to that absorbed.
Table 28-1: Color and Wavelength


Clearly, the color perceived, its brightness and its intensity, depends on the shape of the electronic spectral curve of the absorbing substance, which in turn depends on the chemical structure of the substance. A change in absorption from the blue to the red end of the spectrum corresponds to a decrease in the energy of the associated electronic transitions. We know also that this trend is associated with increasing conjugation of multiple bonds. For instance, 1,2-diphenylethene is colorless, whereas 1,10-diphenyl-1,3,5,7,9-decapentaene is yellow-orange:
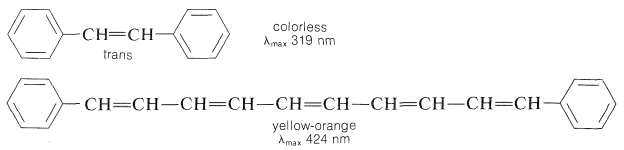

The effect of substituents on colors associated with conjugated systems is of particular interest in the study of dyes, because most dyes have relatively short conjugated systems and would not be intensely colored in the absence of substituent groups. (The plant pigments \(\beta\)-carotene, Section 2-1, and lycopene, often used as food coloring, are exceptions.)
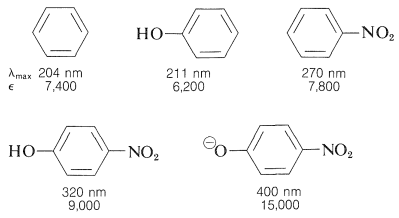
The conjugated \(\pi\) system common to all four compounds is that of the benzenoid ring, which is described as the absorbing chromophore (Section 22-3B). The hydroxyl and nitro substituents can be seen individually to shift the \(\lambda_\text{max}\) of the chromophore to longer wavelengths. However, the combined effect of the two substituents is much more dramatic, especially if the \(\ce{OH}\) group is converted to the corresponding anion, 4-nitrobenzenolate. Now \(\lambda_\text{max}\) is shifted into the visible region, giving a yellow color, and because \(\epsilon\) is large, the color is intense. Thus, properly chosen substituents can shift the main benzenoid absorption band from the ultraviolet into the visible region of the spectrum. Such substituents are often called auxochromes. They act by extending the conjugation of the chromophore and are particularly effective in causing large shifts towards the visible when one substituent is a \(\pi\)-electron donor and the other a \(\pi\)-electron acceptor. Thus, with the 4-nitrobenzenolate ion, interaction between the strongly electron-donating \(\ce{-O}^\ominus\) group and the strongly electron-accepting \(\ce{-NO_2}\) group provides significant stabilization:
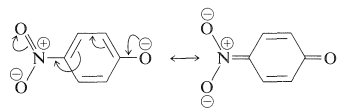
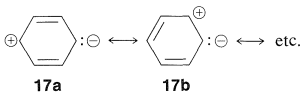
Hence, substitution of an electron-attracting group (such as \(\ce{NO_2}\)) at one end of such a system and an electron-donating group (such as \(\ce{O}^\ominus\)) at the other end should be particularly favorable for stabilization of the excited state (relative to the ground state, where \(17a\), \(17b\), etc., are of lesser importance). At the same time, we should not expect that two electron-attracting (or two electron-donating) groups at opposite ends would be nearly as effective.
We hope you will understand from the foregoing discussion why it is that many intensely colored substances of natural or synthetic origin have conjugated structures with substituents, often cationic or anionic substituents, that can donate or accept electrons from the conjugated system. Such compounds provide us with many useful dyes, pigments, indicators, and food-coloring agents, as well as conferring color on plants and animals. A few examples follow:
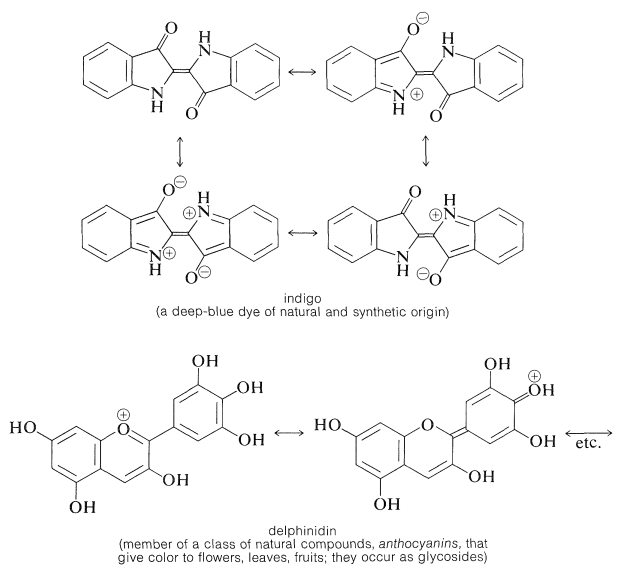
Dyes
Historically, the dye industry has been closely linked with the development of synthetic organic chemistry. Although dyes have been extracted from natural sources for centuries, it was not until 1856 that a synthetic dye was produced commercially. The previous year, William Henry Perkin - at age 17 - oxidized benzenamine (aniline) with potassium dichromate and isolated from the product (which was mostly aniline black; Section 23-11D) a purple compound that was excellent for dyeing silk. Perkin started commercial production of the dye under enormous difficulties. Because there was no organic chemical industry at the time, he had to design and build his own equipment as well as devise efficient syntheses for starting materials. His route to benzenamine stared with crude benzene from coal, which he nitrated and then reduced with iron and acid. He had to make the nitric acid (from nitrate salts and sulfuric acid) because concentrated nitric acid was not available. It was not until 1890 that the structure of Perkin's dye, called mauveine, was established by Otto Fischer. The dye was actually a mixture because the benzene used contained methylbenzene), but the product from the oxidation of benzenamine itself is structurally related to aniline black:
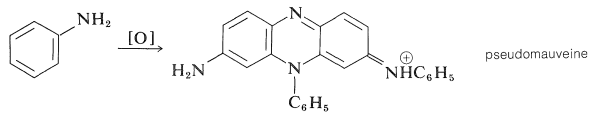
Although the mauveine dyes have been replaced with better dyes, they are representative of a group of useful dyes having the general structure
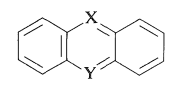
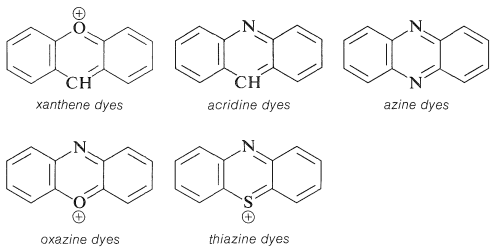
in which \(\ce{X}\) and \(\ce{Y}\) can be oxygen, nitrogen, sulfur, or carbon. The rings invariably carry substituents (hydroxyl or amino) that provide enhanced stabilization of the excited states. Examples of these ring systems follow:
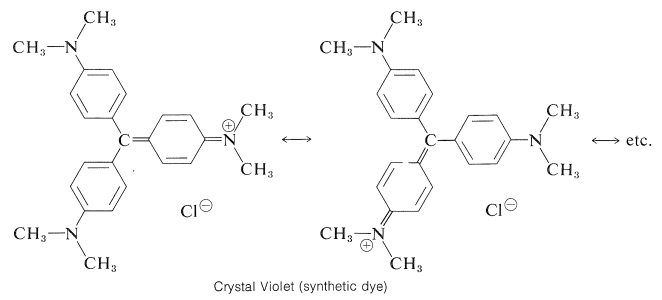
A large number of useful dyes are substituted triphenylmethane derivatives. Crystal Violet (Section 28-4) and phenolphthalein are excellent examples of this kind of dye.
Other important dyes are derivatives of the following types of substances:
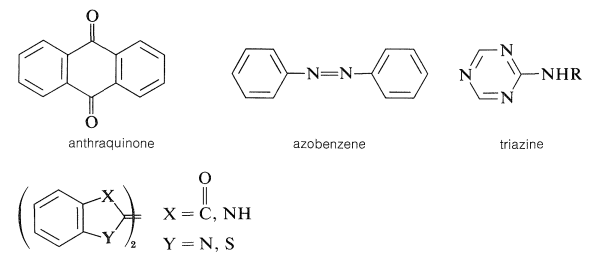
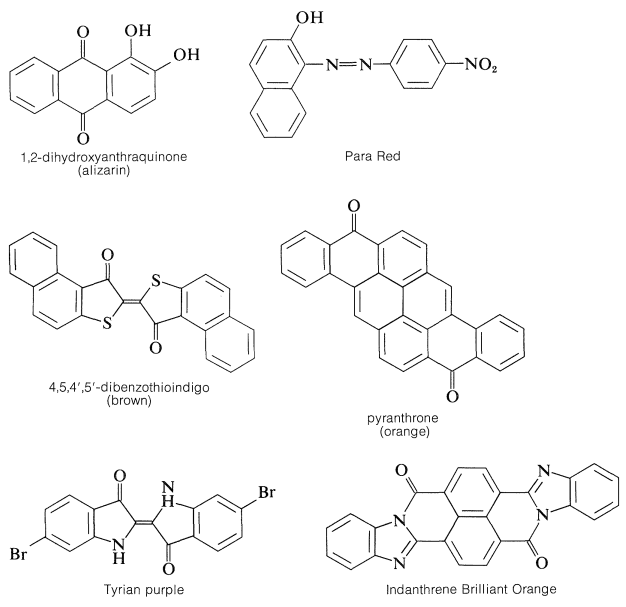
Examples are
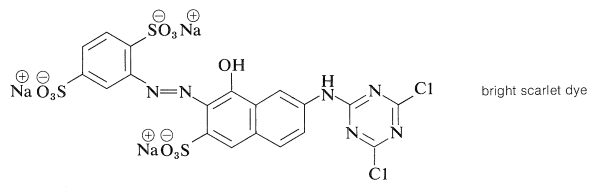
There is more to a successful dye than just an attractive color.\(^5\) If it is to be useful, say for coloring fabrics, some simple means must be available for introducing the color into the fiber and then, usually of greater difficulty and importance, the color must be reasonably permanent - that is, resistant to normal laundry or cleaning procedures (wash-fast) and stable to light (light-fast). Here again, fundamentally important problems are involved. The scientific approach to improving wash-fastness of fabric dyes has to be based on a knowledge of the structural factors bearing on the intermolecular forces that determine solubilities. Light-fastness is connected with the photochemistry of organic compounds.
Pigments
The distinction between a dye and a pigment is that a dye actually is absorbed by the material to be colored, whereas a pigment is applied with a binding material to the surface. Pigments usually are highly insoluble substances. Many insoluble inorganic substances that would be wholly unsatisfactory as dyes are useful pigments.
Copper phthalocyanine is an example of a very important class of organic pigments. These are tetraazatetrabenzo derivatives of the porphyrin compounds discussed in Sections 20-9 and 25-8B. Copper phthalocyanine arises from condensation of four molecules of 1,2-benzenedicarbonitrile in the presence of copper metal at \(200^\text{o}\):

\(^5\)For a good account of dyes, see L. F. Fieser and M. Fieser, Organic Chemistry, D. C. Health & Co., Lexington, Mass., 1956, Chapter 36.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


