5.1: Chemical Equations
- Page ID
- 86210
Learning Objectives
- Define chemical reaction.
- Understand the Law of Conservation of Matter
Water (H2O) is composed of hydrogen and oxygen. Suppose we imagine a process in which we take some elemental hydrogen (H2) and elemental oxygen (O2) and let them react to make water. The statement
"hydrogen and oxygen react to make water"
is one way to represent that process, which is called a chemical reaction. Figure \(\PageIndex{1}\) shows a rather dramatic example of this very reaction.

To simplify the writing of reactions, we use formulas instead of names when we describe a reaction. We can also use symbols to represent other words in the reaction. A plus sign connects the initial substances (and final substances, if there is more than one), and an arrow (→) represents the chemical change:
\[\ce{H_2 + O_2 \rightarrow H_2O} \label{Eq1}\]
This statement is one example of a chemical equation, an abbreviated way of using symbols to represent a chemical change. The substances on the left side of the arrow are called reactants, and the substances on the right side of the arrow are called products. It is not uncommon to include a phase label with each formula—(s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows:
\[\ce{H2(g) + O2(g) \rightarrow H2O (ℓ)} \label{Eq2}\]
This equation is still not complete because it does not satisfy the law of conservation of matter. Count the number of atoms of each element on each side of the arrow. On the reactant side, there are two H atoms and two O atoms; on the product side, there are two H atoms and only one oxygen atom. The equation is not balanced because the number of oxygen atoms on each side is not the same (Figure \(\PageIndex{2}\)).
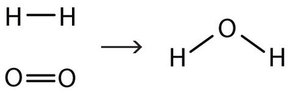
To make this chemical equation conform to the law of conservation of matter, we must revise the amounts of the reactants and the products as necessary to get the same number of atoms of a given element on each side. Because every substance has a characteristic chemical formula, we cannot change the chemical formulas of the individual substances. For example, we cannot change the formula for elemental oxygen to O. However, we can assume that different numbers of reactant molecules or product molecules may be involved. For instance, perhaps two water molecules are produced, not just one:
\[\ce{H2(g) + O2 (g) \rightarrow 2H2O (ℓ)} \label{Eq3}\]
The 2 preceding the formula for water is called a coefficient. It implies that two water molecules are formed. There are now two oxygen atoms on each side of the equation.
This point is so important that we should repeat it. You cannot change the formula of a chemical substance to balance a chemical reaction! You must use the proper chemical formula of the substance.
Unfortunately, by inserting the coefficient 2 in front of the formula for water, we have also changed the number of hydrogen atoms on the product side as well. As a result, we no longer have the same number of hydrogen atoms on each side. This can be easily fixed, however, by putting a coefficient of 2 in front of the diatomic hydrogen reactant:
\[\ce{2H2(g) + O2(g) \rightarrow 2H2O (ℓ)} \label{Eq4}\]
Now we have four hydrogen atoms and two oxygen atoms on each side of the equation. The law of conservation of matter is satisfied because we now have the same number of atoms of each element in the reactants and in the products. We say that the reaction is now balanced (Figure \(\PageIndex{3}\)). Note: The diatomic oxygen has a coefficient of 1, which typically is not written but assumed in balanced chemical equations.
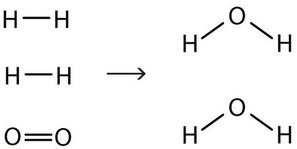
Proper chemical equations should be balanced. Writing balanced reactions is a chemist’s way of acknowledging the law of conservation of matter.
Example \(\PageIndex{1}\)
Is each chemical equation balanced?
- 2Na(s) + O2(g) → 2Na2O(s)
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ)
- AgNO3(aq) + 2KCl(aq) → AgCl(s) + KNO3(aq)
Solution
- By counting, we find two sodium atoms and two oxygen atoms in the reactants and four sodium atoms and two oxygen atoms in the products. This equation is not balanced.
- The reactants have one carbon atom, four hydrogen atoms, and four oxygen atoms. The products have one carbon atom, four hydrogen atoms, and four oxygen atoms. This equation is balanced.
- The reactants have one silver atom, one nitrogen atom, three oxygen atoms, two potassium atoms, and two chlorine atoms. The products have one silver atom, one chlorine atom, one potassium atom, one nitrogen atom, and three oxygen atoms. Because there are different numbers of chlorine and potassium atoms, this equation is not balanced.
Exercise \(\PageIndex{1}\)
Is each chemical equation balanced?
- \(2Hg_{(ℓ)} + O_{2(g)} \rightarrow Hg_2O_{2(s)}\)
- \(C_2H_{4(g)} + 2O_{2(g)} \rightarrow 2CO_{2(g)} + 2H_2O_{(ℓ)}\)
- \(Mg(NO_3)_{2(s)} + 2Li_{(s)} \rightarrow Mg_{(s)} + 2LiNO_{3(s)}\).
- Answer a:
-
balanced
- Answer b:
-
O is not balanced; the 4 atoms of oxygen on the left does not balance with the 6 oxygen atoms on the right
- Answer c:
-
balanced

