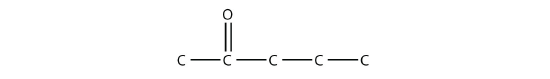15.1: The Carbonyl Group
- Page ID
- 86286
- Identify the aldehyde and ketone functional groups.
There are other functional groups that contain O atoms. Before we introduce them, we define the carbonyl group, which is formed when an O atom and a C atom are joined by a double bond:
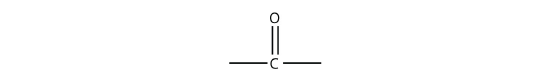
The other two bonds on the C atom are attached to other atoms. It is the identities of these other atoms that define what specific type of compound an organic molecule is.
If one bond of the carbonyl group is made to an H atom, then the molecule is classified as an aldehyde (If there are two H atoms, there is only 1 C atom). When naming aldehydes, the main chain of C atoms must include the carbon in the carbonyl group, which is numbered as position 1 in the carbon chain. The parent name of the hydrocarbon is used, but the suffix -al is appended. (Do not confuse -al with -ol, which is the suffix used for alcohols.) So we have
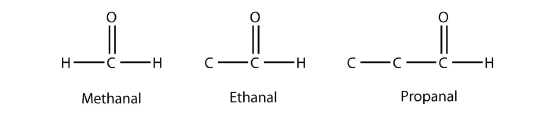
Methanal has a common name with which you may be familiar: formaldehyde. The main thing to note about aldehydes is that the carbonyl group is at the end of a carbon chain.
A carbonyl group in the middle of a carbon chain implies that both remaining bonds of the carbonyl group are made to C atoms. This type of molecule is called a ketone. Despite the fact that aldehydes and ketones have the same carbonyl group, they have different chemical and physical properties and are properly grouped as two different types of compounds. The smallest ketone has three C atoms in it. When naming a ketone, we take the name of the parent hydrocarbon and change the suffix to -one:
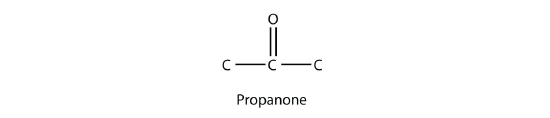
The common name for propanone is acetone. With larger ketones, we must use a number to indicate the position of the carbonyl group, much like a number is used with alkenes and alkynes:
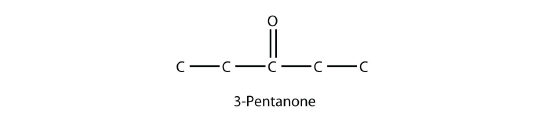
There is another way to name ketones: name the alkyl groups that are attached to the carbonyl group and add the word ketone to the name. So propanone can also be called dimethyl ketone, while 2-butanone is called methyl ethyl ketone.
Draw the structure of 2-pentanone.
Solution
This molecule has five C atoms in a chain, with the carbonyl group on the second C atom. Its structure is as follows: