14.9: Halogen-Containing Compounds
- Page ID
- 86739
- Identify and name a simple alkyl halide.
The presence of a halogen atom (F, Cl, Br, or I; also, X is used to represent any halogen atom) is one of the simplest functional groups. Organic compounds that contain a halogen atom are called alkyl halides. We have already seen some examples of alkyl halides when the addition of halogens across double and triple bonds was introduced in Section 16.3 - "Branched Hydrocarbons;" the products of these reactions were alkyl halides.
A simple alkyl halide can be named like an ionic salt, first by stating the name of the parent alkane as a substituent group (with the -yl suffix) and then the name of the halogen as if it were the anion. So CH3Cl has the common name of methyl chloride, while CH3CH2Br is ethyl bromide and CH3CH2CH2I is propyl iodide. However, this system is not ideal for more complicated alkyl halides.
The systematic way of naming alkyl halides is to name the halogen as a substituent, just like an alkyl group, and use numbers to indicate the position of the halogen atom on the main chain. The name of the halogen as a substituent comes from the stem of the element's name plus the ending -o, so the substituent names are fluoro-, chloro-, bromo- and iodo-. If there is more than one of a certain halogen, we use numerical prefixes to indicate the number of each kind, just as with alkyl groups. For example, this molecule
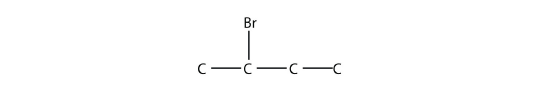
is 2-bromobutane, while this molecule
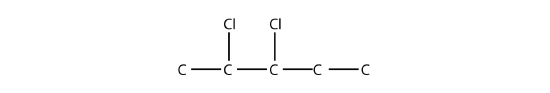
is 2,3-dichloropentane. If alkyl groups are present, the substituents are listed alphabetically. Numerical prefixes are ignored when determining the alphabetical ordering of substituent groups.
Name this molecule.
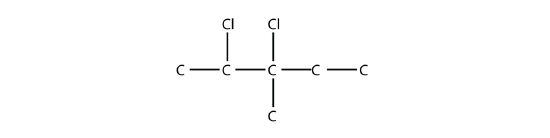
Solution
The longest carbon chain has five C atoms, so the molecule is a pentane. There are two chlorine substituents located on the second and third C atoms, with a one-carbon methyl group on the third C atom as well. The correct name for this molecule is 2,3-dichloro-3-methylpentane.
Name this molecule.
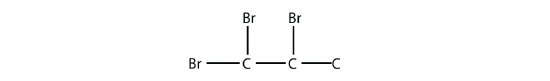
- Answer
-
1,1,2-tribromopropane
Most alkyl halides are insoluble in H2O. Smaller alcohols, however, are very soluble in H2O because these molecules can engage in hydrogen bonding with H2O molecules. For larger molecules, however, the polar OH group is overwhelmed by the nonpolar alkyl part of the molecule. While methanol is soluble in H2O in all proportions, only about 2.6 g of pentanol will dissolve in 100 g of H2O. Larger alcohols have an even lower solubility in H2O.
One reaction common to alcohols and alkyl halides is elimination, the removal of the functional group (either X or OH) and an H atom from an adjacent carbon. The general reaction can be written as follows:
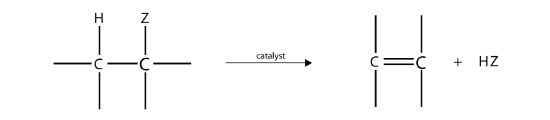
where Z represents either the X or the OH group. The biggest difference between elimination in alkyl halides and elimination in alcohols is the identity of the catalyst: for alkyl halides, the catalyst is a strong base; for alcohols, the catalyst is a strong acid. For compounds in which there are H atoms on more than one adjacent carbon, a mixture of products results.
Predict the organic product(s) of this reaction.
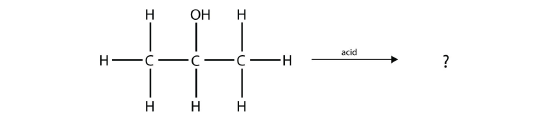
Solution
Under these conditions, an HOH (otherwise known as H2O) molecule will be eliminated, and an alkene will be formed. It does not matter which adjacent carbon loses the H atom; in either case the product will be
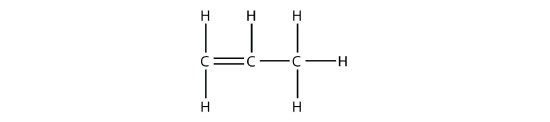
which is propene.
Predict the organic product(s) of this reaction.
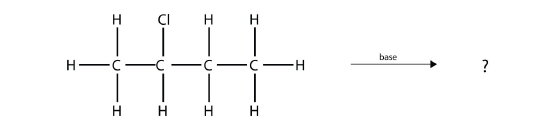
- Answer
-
1-butene and 2-butene
Key Takeaways
- Alkyl halides have a halogen atom as a functional group.
- Alcohols have an OH group as a functional group.
- Nomenclature rules allow us to name alkyl halides and alcohols.
- In an elimination reaction, a double bond is formed as an HX or an HOH molecule is removed.

