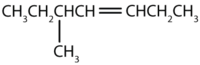13.2: Naming Alkenes and Alkynes
- Page ID
- 86266
- Objective 1
- Objective 2
Here are some basic rules for naming alkenes from the International Union of Pure and Applied Chemistry (IUPAC):
- The longest chain of carbon atoms containing the double bond is considered the parent chain. It is named using the same stem as the alkane having the same number of carbon atoms but ends in -ene to identify it as an alkene. Thus the compound CH2=CHCH3 is propene.
- If there are four or more carbon atoms in a chain, we must indicate the position of the double bond. The carbons atoms are numbered so that the first of the two that are doubly bonded is given the lower of the two possible numbers.The compound CH3CH=CHCH2CH3, for example, has the double bond between the second and third carbon atoms. Its name is 2-pentene (not 3-pentene).
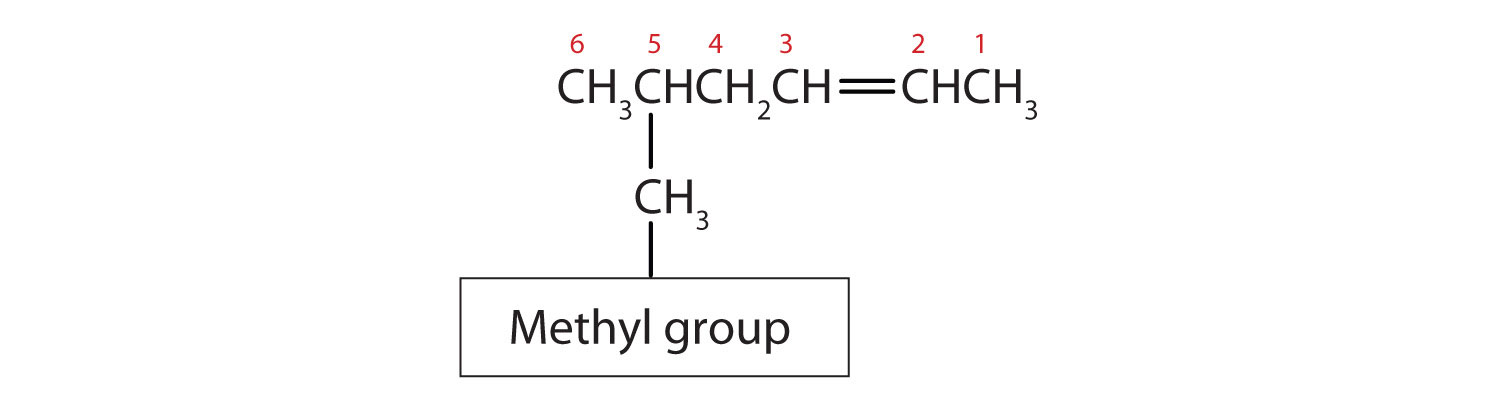
- Substituent groups are named as with alkanes, and their position is indicated by a number. Thus, the structure below is 5-methyl-2-hexene. Note that the numbering of the parent chain is always done in such a way as to give the double bond the lowest number, even if that causes a substituent to have a higher number. The double bond always has priority in numbering.
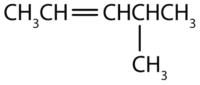
Name each compound.
Solution
- The longest chain containing the double bond has five carbon atoms, so the compound is a pentene (rule 1). To give the first carbon atom of the double bond the lowest number (rule 2), we number from the left, so the compound is a 2-pentene. There is a methyl group on the fourth carbon atom (rule 3), so the compound’s name is 4-methyl-2-pentene.
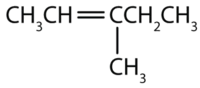
- The longest chain containing the double bond has five carbon atoms, so the parent compound is a pentene (rule 1). To give the first carbon atom of the double bond the lowest number (rule 2), we number from the left, so the compound is a 2-pentene. There is a methyl group on the third carbon atom (rule 3), so the compound’s name is 3-methyl-2-pentene.
Name each compound.
-
CH3CH2CH2CH2CH2CH=CHCH3
Just as there are cycloalkanes, there are cycloalkenes. These compounds are named like alkenes, but with the prefix cyclo- attached to the beginning of the parent alkene name.
Draw the structure for each compound.
- 3-methyl-2-pentene
- cyclohexene
Solution
- First write the parent chain of five carbon atoms: C–C–C–C–C. Then add the double bond between the second and third carbon atoms:
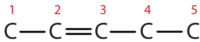
Now place the methyl group on the third carbon atom and add enough hydrogen atoms to give each carbon atom a total of four bonds.
- First, consider what each of the three parts of the name means. Cyclo means a ring compound, hex means 6 carbon atoms, and -ene means a double bond.

Draw the structure for each compound.
- 2-ethyl-1-hexene
- cyclopentene
The IUPAC nomenclature for alkynes is similar to that for alkenes except that the suffix -yne is used to indicate a triple bond in the chain. For example, \(\mathrm{CH_3CH_2C≡CH}\) is called 1-butyne.
Describe the geometry and hybridization of the carbon atoms in the following molecule:
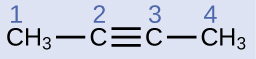
Solution
Carbon atoms 1 and 4 have four single bonds and are thus tetrahedral with sp3 hybridization. Carbon atoms 2 and 3 are involved in the triple bond, so they have linear geometries and would be classified as sp hybrids.
Identify the hybridization and bond angles at the carbon atoms in the molecule shown:
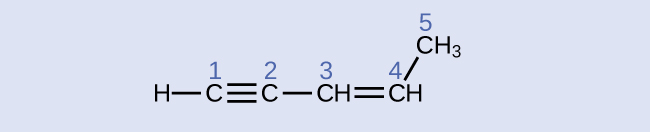
- Answer
-
carbon 1: sp, 180°; carbon 2: sp, 180°; carbon 3: sp2, 120°; carbon 4: sp2, 120°; carbon 5: sp3, 109.5°