11.2: The Discovery and Nature of Radioactivity
- Page ID
- 86252
- To define and give examples of the major types of radioactivity.
Atomic theory in the 19th century presumed that nuclei had fixed compositions. But in 1896, the French scientist Henri Becquerel found that a uranium compound placed near a photographic plate made an image on the plate, even if the compound was wrapped in black cloth. He reasoned that the uranium compound was emitting some kind of radiation that passed through the cloth to expose the photographic plate. Further investigations showed that the radiation was a combination of particles and electromagnetic rays, with its ultimate source as the atomic nucleus. These emanations were ultimately called, collectively, radioactivity.
There are three main forms of radioactive emissions. The first is called an alpha particle,which is symbolized by the Greek letter \(α\). An alpha particle is composed of two protons and two neutrons, and so it is the same as a helium nucleus. (We often use \(\ce{^{4}_{2}He}\) to represent an alpha particle.) It has a 2+ charge. When a radioactive atom emits an alpha particle, the original atom’s atomic number decreases by two (because of the loss of two protons), and its mass number decreases by four (because of the loss of four nuclear particles). We can represent the emission of an alpha particle with a nuclear equation—for example, the alpha-particle emission of uranium-235 is as follows:
\[\ce{^{235}_{92}U \rightarrow \,_2^4He + \, _{90}^{231}Th} \label{Eq2}\]
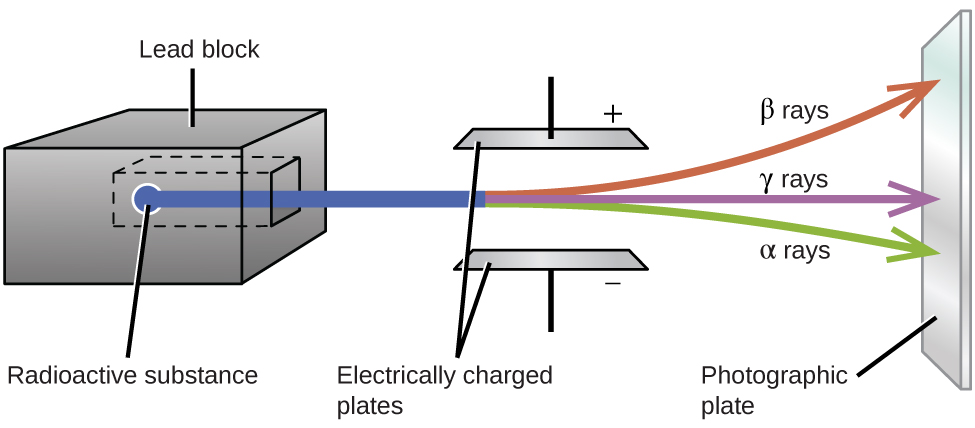
Ernest Rutherford’s experiments involving the interaction of radiation with a magnetic or electric field (Figure \(\PageIndex{1}\)) helped him determine that one type of radiation consisted of positively charged and relatively massive \(α\) particles; a second type was made up of negatively charged and much less massive \(β\) particles; and a third was uncharged electromagnetic waves, \(γ\) rays. We now know that \(α\) particles are high-energy helium nuclei, \(β\) particles are high-energy electrons, and \(γ\) radiation compose high-energy electromagnetic radiation. We classify different types of radioactive decay by the radiation produced.
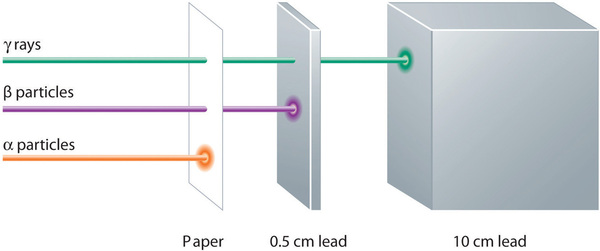
Alpha, beta, and gamma emissions have different abilities to penetrate matter (Figure \(\PageIndex{2}\)). The relatively large alpha particle is easily stopped by matter (although it may impart a significant amount of energy to the matter it contacts). Beta particles penetrate slightly into matter, perhaps a few centimeters at most. Gamma rays can penetrate deeply into matter and can impart a large amount of energy into the surrounding matter. Table \(\PageIndex{1}\) summarizes the properties of the three main types of radioactive emissions.
| Characteristic | Alpha Particles | Beta Particles | Gamma Rays |
|---|---|---|---|
| symbols | α, \(\mathrm{_{2}^{4}He}\) | β, \(\ce{^{0}_{-1} e}\) | γ |
| identity | helium nucleus | electron | electromagnetic radiation |
| charge | 2+ | 1− | none |
| mass number | 4 | 0 | 0 |
| penetrating power | minimal (will not penetrate skin) | short (will penetrate skin and some tissues slightly) | deep (will penetrate tissues deeply) |
Key Takeaway
The major types of radioactivity include alpha particles, beta particles, and gamma rays.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).

