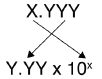10.6: Working with pH
- Page ID
- 86696
- Calculate pH from \([H_3O^+]\) and \([H_3O^+]\) from pH.
Calculating pH from Hydronium Concentration
The pH of solutions can be determined by using logarithms as illustrated in the next example for stomach acid. Stomach acid is a solution of \(HCl\) with a hydronium ion concentration of \(1.2 \times 10^{−3}\; M\), what is the \(pH\) of the solution?
\[ \begin{align} \mathrm{pH} &= \mathrm{-\log [H_3O^+]} \nonumber \\ &=-\log(1.2 \times 10^{−3}) \nonumber \\ &=−(−2.92)=2.92 \nonumber \end{align}\]
To get the log value on your calculator, enter the number (in this case, the hydronium ion concentration) first, then press the LOG key.
If the number is 1.0 x 10-5 (for [H3O+] = 1.0 x 10-5 M) you should get an answer of "-5".
If you get a different answer, or an error, try pressing the LOG key before you enter the number.
Find the pH, given the \([H_3O^+]\) of the following:
- 1 ×10-3 M
- 2.5 ×10-11 M
- 4.7 ×10-9 M
Solution
|
Steps for Problem Solving |
|
|---|---|
| Identify the "given" information and what the problem is asking you to "find." |
Given:
Find: ? pH |
| Plan the problem. |
Need to use the expression for pH (Equation \ref{pH}). pH = - log [H3O+] |
| Calculate. |
Now substitute the known quantity into the equation and solve.
The other issue that concerns us here is significant figures. Because the number(s) before the decimal point in a logarithm relate to the power on 10, the number of digits after the decimal point is what determines the number of significant figures in the final answer:
|
Find the pH, given [H3O+] of the following:
- 5.8 ×10-4 M
- 1.0×10-7
- Answer a
- 3.22
- Answer b
- 7.00
Calculating Hydronium Concentration from pH
Sometimes you need to work "backwards"—you know the pH of a solution and need to find \([H_3O^+]\), or even the concentration of the acid solution. How do you do that? To convert pH into \([H_3O^+]\) we solve Equation \ref{pH} for \([H_3O^+]\). This involves taking the antilog (or inverse log) of the negative value of pH .
\[[\ce{H3O^{+}}] = \text{antilog} (-pH)\]
or
\[[\ce{H_3O^+}] = 10^{-pH} \label{ph1}\]
As mentioned above, different calculators work slightly differently—make sure you can do the following calculations using your calculator.
We have a solution with a pH = 8.3. What is [H3O+] ?
With some calculators you will do things in the following order:
- Enter 8.3 as a negative number (use the key with both the +/- signs, not the subtraction key).
- Use your calculator's 2nd or Shift or INV (inverse) key to type in the symbol found above the LOG key. The shifted function should be 10x.
- You should get the answer 5.0 × 10-9.
Other calculators require you to enter keys in the order they appear in the equation.
- Use the Shift or second function to key in the 10x function.
- Use the +/- key to type in a negative number, then type in 8.3.
- You should get the answer 5.0 × 10-9.
If neither of these methods work, try rearranging the order in which you type in the keys. Don't give up—you must master your calculator!
Find the hydronium ion concentration in a solution with a pH of 12.6. Is this solution an acid or a base? How do you know?
Solution
| Steps for Problem Solving | |
|---|---|
| Identify the "given" information and what the problem is asking you to "find." |
Given: pH = 12.6 Find: [H3O+] = ? M |
| Plan the problem. |
Need to use the expression for [H3O+] (Equation \ref{ph1}). [H3O+] = antilog (-pH) or [H3O+] = 10-pH |
| Calculate. |
Now substitute the known quantity into the equation and solve. [H3O+] = antilog (12.60) = 2.5 x 10-13 M (2 significant figures since 4.7 has 12.60 2 decimal places) or [H3O+] = 10-12.60 = 2.5 x 10-13 M (2 significant figures since 4.7 has 12.60 2 decimal places) The other issue that concerns us here is significant figures. Because the number(s) before the decimal point in a logarithm relate to the power on 10, the number of digits after the decimal point is what determines the number of significant figures in the final answer:
|
If moist soil has a pH of 7.84, what is [H3O+] of the soil solution?
- Answer
- 1.5 x 10-8 M