10.2: Acid and Base Strength
- Page ID
- 86247
- Define a strong and a weak acid and base.
- Recognize an acid or a base as strong or weak.
Strong and Weak Acids
Except for their names and formulas, so far we have treated all acids as equals, especially in a chemical reaction. However, acids can be very different in a very important way. Consider \(\ce{HCl(aq)}\). When \(\ce{HCl}\) is dissolved in \(\ce{H2O}\), it completely dissociates (separates) into H+(aq) and Cl−(aq) ions; all the HCl molecules become ions:
\[\ce {HCl\ \overset{100\%}{\rightarrow}H^{+}(aq) + Cl^{-}(aq)} \]
Any acid that dissociates 100% into ions is called a strong acid. If it does not dissociate 100%, it is a weak acid. HC2H3O2 is an example of a weak acid:
\[HC_{2}H_{3}O_{2}\ \overset{\sim 5\%}{\longrightarrow}H^{+}(aq)+C_{2}H_{3}O_{2}^{-}(aq)\]
Because this reaction does not go 100% to completion, it is more appropriate to write it as a reversible reaction:
\[HC_{2}H_{3}O_{2}\rightleftharpoons H^{+}(aq)+C_{2}H_{3}O_{2}^{-}(aq)\]
As it turns out, there are very few strong acids, which are given in Table \(\PageIndex{1}\). If an acid is not listed here, it is a weak acid. It may be 1% ionized or 99% ionized, but it is still classified as a weak acid.
Any acid that dissociates 100% into ions is called a strong acid. If it does not dissociate 100%, it is a weak acid.
| Strong Acids | Strong Bases | ||
|---|---|---|---|
| \(\ce{HClO4}\) | perchloric acid | \(\ce{LiOH}\) | lithium hydroxide |
| \(\ce{HCl}\) | hydrochloric acid | \(\ce{NaOH}\) | sodium hydroxide |
| \(\ce{HBr}\) | hydrobromic acid | \(\ce{KOH}\) | potassium hydroxide |
| \(\ce{HI}\) | hydroiodic acid | \(\ce{Ca(OH)2}\) | calcium hydroxide |
| \(\ce{HNO3}\) | nitric acid | \(\ce{Sr(OH)2}\) | strontium hydroxide |
| \(\ce{H2SO4}\) | sulfuric acid | \(\ce{Ba(OH)2}\) | barium hydroxide |
Strong and Weak Bases
The issue is similar with bases: a strong base is a base that is 100% ionized in solution. If it is less than 100% ionized in solution, it is a weak base. There are very few strong bases (Table \(\PageIndex{1}\)); any base not listed is a weak base. All strong bases are OH– compounds. So a base based on some other mechanism, such as NH3 (which does not contain OH− ions as part of its formula), will be a weak base.
Identify each acid or base as strong or weak.
- HCl
- Mg(OH)2
- C5H5N
Solution
- Because HCl is listed in Table \(\PageIndex{1}\), it is a strong acid.
- Because Mg(OH)2 is listed in Table \(\PageIndex{1}\), it is a strong base.
- The nitrogen in C5H5N would act as a proton acceptor and therefore can be considered a base, but because it does not contain an OH compound, it cannot be considered a strong base; it is a weak base.
Identify each acid or base as strong or weak.
- \(\ce{RbOH}\)
- \(\ce{HNO_2}\)
- Answer
-
- strong base
- weak acid
Write the balanced chemical equation for the dissociation of Ca(OH)2 and indicate whether it proceeds 100% to products or not.
Solution
This is an ionic compound of Ca2+ ions and OH− ions. When an ionic compound dissolves, it separates into its constituent ions:
\[\ce{Ca(OH)2 → Ca^{2+}(aq) + 2OH^{−}(aq)} \nonumber\]
Because Ca(OH)2 is listed in Table \(\PageIndex{1}\), this reaction proceeds 100% to products.
Write the balanced chemical equation for the dissociation of hydrazoic acid (HN3) and indicate whether it proceeds 100% to products or not.
- Answer a
- The reaction is as follows:
- \[\ce{HN3 → H^{+}(aq) + N3^{−}(aq)} \nonumber\]
- It does not proceed 100% to products because hydrazoic acid is not a strong acid.
Many household products are acids or bases. For example, the owner of a swimming pool may use muriatic acid to clean the pool. Muriatic acid is another name for hydrochloric acid [\(\ce{HCl(aq)}\)]. Vinegar has already been mentioned as a dilute solution of acetic acid [\(\ce{HC2H3O2(aq)}\)]. In a medicine chest, one may find a bottle of vitamin C tablets; the chemical name of vitamin C is ascorbic acid (\(\ce{HC6H7O6}\)).
One of the more familiar household bases is ammonia (\(\ce{NH3}\)), which is found in numerous cleaning products. As we mentioned previously, ammonia is a base because it increases the hydroxide ion concentration by reacting with water:
\[\ce{NH3(aq) + H2O(ℓ) \rightarrow NH^{+}4(aq) + OH^{−}(aq)} \label{Eq3} \]
Many soaps are also slightly basic because they contain compounds that act as Brønsted-Lowry bases, accepting protons from water and forming excess hydroxide ions. This is one reason that soap solutions are slippery.

Perhaps the most dangerous household chemical is the lye-based drain cleaner. Lye is a common name for sodium hydroxide, although it is also used as a synonym for potassium hydroxide. Lye is an extremely caustic chemical that can react with grease, hair, food particles, and other substances that may build up and form a clog in a pipe. Unfortunately, lye can also attack tissues and other substances in our bodies. Thus, when we use lye-based drain cleaners, we must be very careful not to touch any of the solid drain cleaner or spill the water it was poured into. Safer, nonlye drain cleaners use peroxide compounds to react on the materials in the clog and clear the drain.
Chemical Equilibrium in Weak Acids and Bases
Ionization of weak acids or bases are reversible reactions, which means the forward and reverse reactions occur and eventually reach equilbrium. For example, the ionization of the weak acid \(\ce{HC2H3O2(aq)}\) is as follows:
\[\ce{HC2H3O2(aq) + H2O(ℓ) \rightarrow H3O^{+}(aq) + C2H3O^{−}2(aq)} \label{Eq4} \]
The reverse process also begins to occur:
\[\ce{H3O^{+}(aq) + C2H3O^{−}2(aq) \rightarrow HC2H3O2(aq) + H2O(ℓ)} \label{Eq5} \]
Eventually, there is a balance between the two opposing processes, and no additional change occurs. The chemical reaction is better represented at this point with a double arrow:
\[\ce{HC2H3O2(aq) + H2O(ℓ) <=> H3O^{+}(aq) + C2H3O^{-}2(aq)} \label{Eq6} \]
The \(\rightleftharpoons\) implies that both the forward and reverse reactions are occurring, and their effects cancel each other out. A process at this point is considered to be at chemical equilibrium (or equilibrium). It is important to note that the processes do not stop. They balance out each other so that there is no further net change; that is, chemical equilibrium is a dynamic equilibrium.
Write the equilibrium chemical equation for the partial ionization of each weak acid or base.
- HNO2(aq)
- C5H5N(aq)
Solution
- HNO2(aq) + H2O(ℓ) ⇆ NO2−(aq) + H3O+(aq)
- C5H5N(aq) + H2O(ℓ) ⇆ C5H5NH+(aq) + OH−(aq)
Write the equilibrium chemical equation for the partial ionization of each weak acid or base.
- \(HF_{(aq)}\)
- \(AgOH_{(aq)}\)
- CH3NH2(aq)
- Answer
-
a. HF(aq) + H2O(ℓ) ⇆ F−(aq) + H3O+(aq)
b. AgOH(aq) ⇆ Ag+(aq) + OH−(aq)
c. CH3NH2(aq) + H2O(ℓ) ⇆ CH3NH3+(aq) + OH−(aq)
Strengths of Conjugate Acid and Base Pairs
The extent to which an acid, \(\ce{HA}\), donates protons to water molecules depends on the strength of the conjugate base, \(\ce{A^{−}}\), of the acid. If \(\ce{A^{−}}\) is a strong base, any protons that are donated to water molecules are recaptured by \(\ce{A^{−}}\). Thus there is relatively little \(\ce{A^{−}}\) and \(\ce{H3O+}\) in solution, and the acid, \(\ce{HA}\), is weak. If \(\ce{A^{−}}\) is a weak base, water binds the protons more strongly, and the solution contains primarily \(\ce{A^{−}}\) and \(\ce{H3O^{+}}\)—the acid is strong. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (Figure \(\PageIndex{2}\)).
The first six acids in Figure \(\PageIndex{2}\) are the most common strong acids. These acids are completely dissociated in aqueous solution. The conjugate bases of these acids are weaker bases than water. When one of these acids dissolves in water, their protons are completely transferred to water, the stronger base.
Those acids that lie between the hydronium ion and water in Figure \(\PageIndex{2}\) form conjugate bases that can compete with water for possession of a proton. Both hydronium ions and non-ionized acid molecules are present in equilibrium in a solution of one of these acids. Compounds that are weaker acids than water (those found below water in the column of acids) in Figure \(\PageIndex{2}\) exhibit no observable acidic behavior when dissolved in water. Their conjugate bases are stronger than the hydroxide ion, and if any conjugate base were formed, it would react with water to re-form the acid.
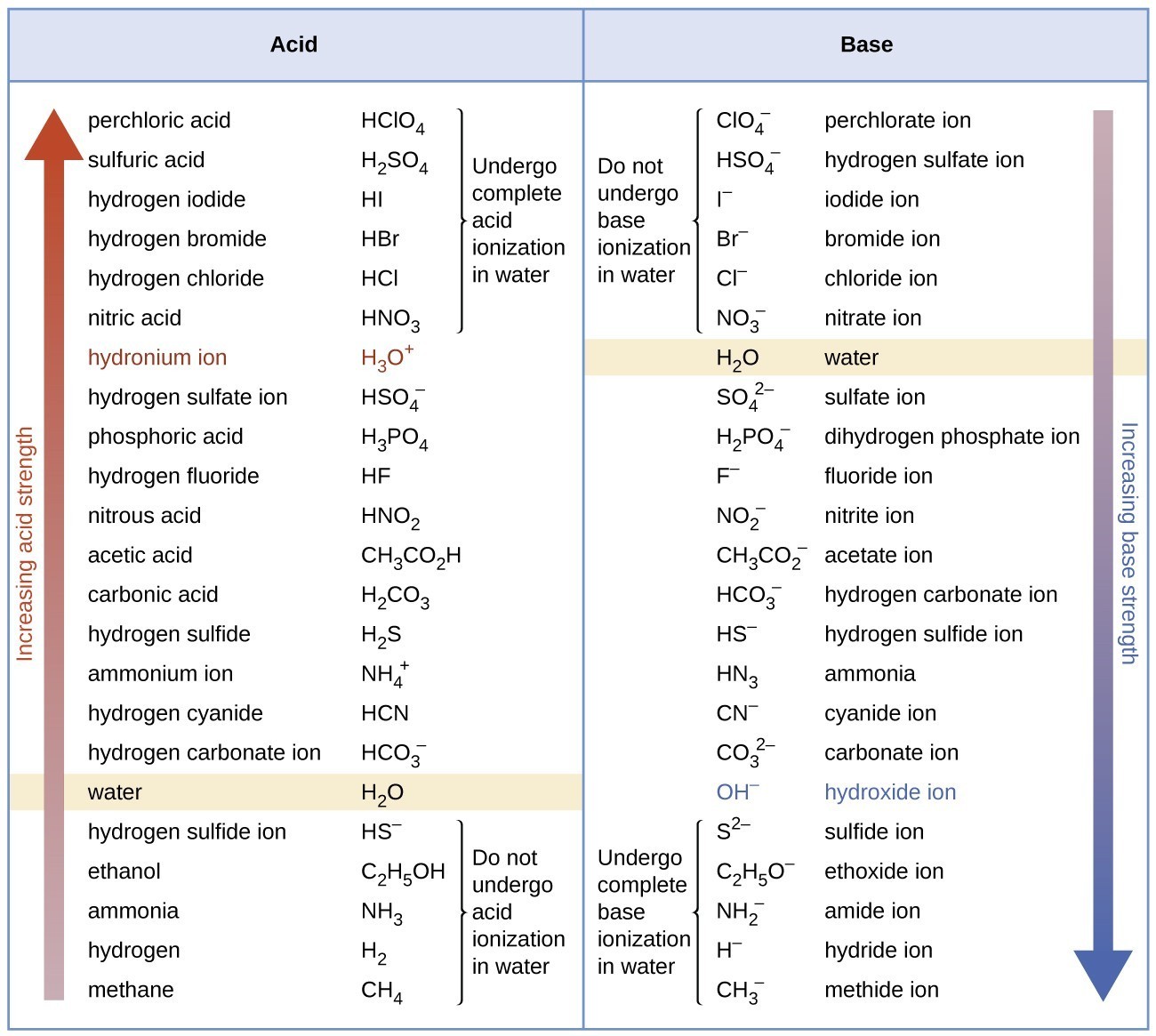
The extent to which a base forms hydroxide ion in aqueous solution depends on the strength of the base relative to that of the hydroxide ion, as shown in the last column in Figure \(\PageIndex{2}\). A strong base, such as one of those lying below hydroxide ion, accepts protons from water to yield 100% of the conjugate acid and hydroxide ion. Those bases lying between water and hydroxide ion accept protons from water, but a mixture of the hydroxide ion and the base results. Bases that are weaker than water (those that lie above water in the column of bases) show no observable basic behavior in aqueous solution.
Key Takeaways
- Strong acids and bases are 100% ionized in aqueous solution.
- Weak acids and bases are less than 100% ionized in aqueous solution.

