1.6: Physical Quantities - Units and Scientific Notation
- Page ID
- 86581
- Express quantities properly using a number and a unit.
- Recognize the different measurement systems used in chemistry.
- Describe how prefixes are used in the metric system and identify how the prefixes milli-, centi-, and kilo- compare to the base unit.
- Understand when and how to use scientific notation to represent measurements.
Measurements
A coffee maker’s instructions tell you to fill the coffeepot with 4 cups of water and use 3 scoops of coffee. When you follow these instructions, you are measuring. When you visit a doctor’s office, a nurse checks your temperature, height, weight, and perhaps blood pressure (Figure \(\PageIndex{1}\)); the nurse is also measuring.
Chemists measure the properties of matter using a variety of devices or measuring tools, many of which are similar to those used in everyday life. Rulers are used to measure length, balances (scales) are used to measure mass (weight), and graduated cylinders or pipettes are used to measure volume. Measurements made using these devices are expressed as quantities. A quantity is an amount of something and consists of a number and a unit. The number tells us how many (or how much), and the unit tells us what the scale of measurement is. For example, when a distance is reported as “5.2 kilometers,” we know that the quantity has been expressed in units of kilometers and that the number of kilometers is 5.2.
\[\color{red} \underbrace{5.2}_{\text{number}} \color{blue} \underbrace{\text{kilometers}}_{\text{unit}} \nonumber\]
If you ask a friend how far he or she walks from home to school, and the friend answers “12” without specifying a unit, you do not know whether your friend walks—for example, 12 miles, 12 kilometers, 12 furlongs, or 12 yards.
Without units, a number can be meaningless, confusing, or possibly life threatening. Suppose a doctor prescribes phenobarbital to control a patient’s seizures and states a dosage of “100” without specifying units. Not only will this be confusing to the medical professional giving the dose, but the consequences can be dire: 100 mg given three times per day can be effective as an anticonvulsant, but a single dose of 100 g is more than 10 times the lethal amount.
Both a number and a unit must be included to express a quantity properly.
To understand chemistry, we need a clear understanding of the units chemists work with and the rules they follow for expressing numbers.
Identify the number and the unit in each quantity.
- one dozen eggs
- 2.54 centimeters
- a box of pencils
- 88 meters per second
Answers
- The number is one, and the unit is dozen.
- The number is 2.54, and the unit is centimeter.
- The number 1 is implied because the quantity is only a box. The unit is box of pencils.
- The number is 88, and the unit is meters per second. Note that in this case the unit is actually a combination of two units: meters and seconds.
Identify the number and the unit in each quantity.
- 99 bottles of soda
- 60 miles per hour
- 32 fluid ounces
- 98.6 degrees Fahrenheit
- Answer a
-
The number is 99, and the unit is bottles of soda.
- Answer b
-
The number is 60, and the unit is miles per hour.
- Answer c
-
The number 32, and the unit is fluid ounces
- Answer d
-
The number is 98.6, and the unit is degrees Fahrenheit
The International System of Units
How long is a yard? It depends on whom you ask and when you asked the question. Today we have a standard definition of the yard, which you can see marked on every football field. If you move the ball ten yards, you get a first down and it doesn't matter whether you are playing in Los Angeles, Dallas, or Green Bay. But at one time that yard was arbitrarily defined as the distance from the tip of the king's nose to the end of his outstretched hand. Of course, the problem there is simple: new king, new distance (and then you have to remark all those football fields).


SI Base Units
All measurements depend on the use of units that are well known and understood. The English system of measurement units (inches, feet, ounces, etc.) are not used in science because of the difficulty in converting from one unit to another. The metric system is used because all metric units are based on multiples of 10, making conversions very simple. The metric system was originally established in France in 1795. The International System of Units is a system of measurement based on the metric system. The acronym SI is commonly used to refer to this system and stands for the French term, Le Système International d'Unités. The SI was adopted by international agreement in 1960 and is composed of seven base units in Table \(\PageIndex{1}\).
| Quantity | SI Base Unit | Symbol |
|---|---|---|
| Length | meter | \(\text{m}\) |
| Mass | kilogram | \(\text{kg}\) |
| Temperature | kelvin | \(\text{K}\) |
| Time | second | \(\text{s}\) |
| Amount of a Substance | mole | \(\text{mol}\) |
| Electric Current | ampere | \(\text{A}\) |
| Luminous Intensity | candela | \(\text{cd}\) |
The first units are frequently encountered in chemistry. All other measurement quantities, such as volume, force, and energy, can be derived from these seven base units.
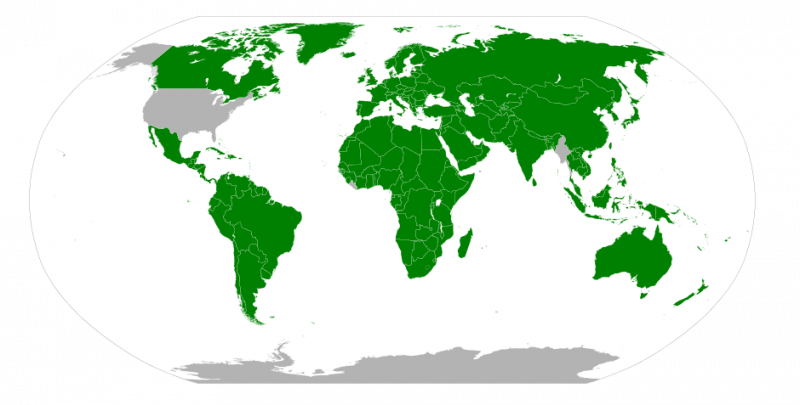
The map below shows the adoption of the SI units in countries around the world. The United States has legally adopted the metric system for measurements, but does not use it in everyday practice. Great Britain and much of Canada use a combination of metric and imperial units.
Metric Prefixes
Conversions between metric system units are straightforward because the system is based on powers of ten. For example, meters, centimeters, and millimeters are all metric units of length. There are 10 millimeters in 1 centimeter and 100 centimeters in 1 meter. Metric prefixes are used to distinguish between units of different size. These prefixes all derive from either Latin or Greek terms. For example, mega comes from the Greek word \(\mu \varepsilon \gamma \alpha \varsigma\), meaning "great". Table \(\PageIndex{2}\) lists the most common metric prefixes and their relationship to the central unit that has no prefix. Length is used as an example to demonstrate the relative size of each prefixed unit.
| Prefix | Unit Abbreviation | Meaning | Example |
|---|---|---|---|
| giga | \(\text{G}\) | 1,000,000,000 | 1 gigameter \(\left( \text{Gm} \right)=10^9 \: \text{m}\) |
| mega | \(\text{M}\) | 1,000,000 | 1 megameter \(\left( \text{Mm} \right)=10^6 \: \text{m}\) |
| kilo | \(\text{k}\) | 1,000 | 1 kilometer \(\left( \text{km} \right)=1,000 \: \text{m}\) |
| hecto | \(\text{h}\) | 100 | 1 hectometer \(\left( \text{hm} \right)=100 \: \text{m}\) |
| deka | \(\text{da}\) | 10 | 1 dekameter \(\left( \text{dam} \right)=10 \: \text{m}\) |
| 1 | 1 meter \(\left( \text{m} \right)\) | ||
| deci | \(\text{d}\) | 1/10 | 1 decimeter \(\left( \text{dm} \right)=0.1 \: \text{m}\) |
| centi | \(\text{c}\) | 1/100 | 1 centimeter \(\left( \text{cm} \right)=0.01 \: \text{m}\) |
| milli | \(\text{m}\) | 1/1,000 | 1 millimeter \(\left( \text{mm} \right)=0.001 \: \text{m}\) |
| micro | \(\mu\) | 1/1,000,000 | 1 micrometer \(\left( \mu \text{m} \right)=10^{-6} \: \text{m}\) |
| nano | \(\text{n}\) | 1/1,000,000,000 | 1 nanometer \(\left( \text{nm} \right)=10^{-9} \: \text{m}\) |
| pico | \(\text{p}\) | 1/1,000,000,000,000 | 1 picometer \(\left( \text{pm} \right)=10^{-12} \: \text{m}\) |
Just as expressing a quantity without a unit is meaningless, so is using the incorrect format for units and prefixes. As you may have noticed, most metric abbreviations are lowercase. We use "\(\text{m}\)" for meter and not "\(\text{M}\)", which you will see later to represent solution concentration, something very different from length. However, when it comes to volume, the base unit "liter" is abbreviated as "\(\text{L}\)" and not "\(\text{l}\)". So we would write 3.5 milliliters as \(3.5 \: \text{mL}\).
As a practical matter, whenever possible you should express the units in a small and manageable number. If you are measuring the weight of a material that weighs \(6.5 \: \text{kg}\), this is easier than saying it weighs \(6500 \: \text{g}\) or \(0.65 \: \text{dag}\). All three are correct, but the \(\text{kg}\) units in this case make for a small and easily managed number. However, if a specific problem needs grams instead of kilograms, go with the grams for consistency.
Give the abbreviation for each unit and define the abbreviation in terms of the base unit.
- kiloliter
- microsecond
- decimeter
- nanogram
Solutions
| Explanation | Answer | |
|---|---|---|
| a | The prefix kilo means “1,000 ×,” so 1 kL equals 1,000 L | kL |
| b | The prefix micro implies 1/1,000,000th of a unit, so 1 µs equals 0.000001 s. | µs |
| c | The prefix deci means 1/10th, so 1 dm equals 0.1 m. | dm |
| d | The prefix nano means 1/1000000000, so a nanogram is equal to 0.000000001 g | ng |
Give the abbreviation for each unit and define the abbreviation in terms of the base unit.
- kilometer
- milligram
- nanosecond
- centiliter
- Answer a
- km
- Answer b
- mg
- Answer c
- ns
- Answer d
- cL
Scientific Notation
Chemists often work with numbers that are exceedingly large or small. For example, entering the mass in grams of a hydrogen atom into a calculator would require a display with at least 24 decimal places. A system called scientific notation avoids much of the tedium and awkwardness of manipulating numbers with large or small magnitudes. In scientific notation, these numbers are expressed in the form
\[ N \times 10^n\]
where N is a number greater than or equal to 1 and less than 10 (1 ≤ N < 10), and \(n\) is a positive or negative integer (100 = 1). The number 10 is called the base because it is this number that is raised to the power \(n\). Although a base number may have values other than 10, the base number in scientific notation is always 10.
A simple way to convert numbers to scientific notation is to move the decimal point as many places to the left or right as needed to give a number from 1 to 10 (N). The magnitude of n is then determined as follows:
- If the decimal point is moved to the left \(n\) places, \(n\) is positive.
- If the decimal point is moved to the right \(n\) places, \(n\) is negative.
Another way to remember this is to recognize that as the number N decreases in magnitude, the exponent increases and vice versa. The application of this rule is illustrated in Example \(\PageIndex{1}\).
Convert each number to scientific notation.
- 637.8
- 0.0479
- 7.86
- 12,378
- 0.00032
- 61.06700
- 2002.080
- 0.01020
Solution
| Explanation | Answer | |
|---|---|---|
| a |
To convert 637.8 to a number from 1 to 10, we move the decimal point two places to the left: 637.8 Because the decimal point was moved two places to the left, n = 2. |
\(6.378 \times 10^2\) |
| b |
To convert 0.0479 to a number from 1 to 10, we move the decimal point two places to the right: 0.0479 Because the decimal point was moved two places to the right, n = −2. |
\(4.79 \times 10^{−2}\) |
| c | This is usually expressed simply as 7.86. (Recall that 100 = 1.) | \(7.86 \times 10^0\) |
| d | Because the decimal point was moved four places to the left, n = 4. | \(1.2378 \times 10^4\) |
| e | Because the decimal point was moved four places to the right, n = −4. | \(3.2 \times 10^{−4}\) |
| f | Because the decimal point was moved one place to the left, n = 1. |
\(6.106700 \times 10^1\) |
| g | Because the decimal point was moved three places to the left, n = 3. | \(2.002080 \times 10^3\) |
| h | Because the decimal point was moved two places to the right, n = -2. | \(1.020 \times 10^{−2}\) |
Scientific Notation: Addition and Subtraction
Before numbers expressed in scientific notation can be added or subtracted, they must be converted to a form in which all the exponents have the same value. The appropriate operation is then carried out on the values of N. Example \(\PageIndex{2}\) illustrates how to do this.
Carry out the appropriate operation and then express the answer in scientific notation.
- \( (1.36 \times 10^2) + (4.73 \times 10^3) \nonumber\)
- \((6.923 \times 10^{−3}) − (8.756 \times 10^{−4}) \nonumber\)
Solution
| Explanation | Answer | |
|---|---|---|
| a |
Both exponents must have the same value, so these numbers are converted to either \((1.36 \times 10^2) + (47.3 \times 10^2) = (1.36 + 47.3) \times 10^2 = 48.66 × 10^2\) or \((0.136 \times 10^3) + (4.73 \times 10^3) = (0.136 + 4.73) \times 10^3) = 4.87 \times 10^3\). Choosing either alternative gives the same answer, reported to two decimal places: In converting 48.66 × 102 to scientific notation, \(n\) has become more positive by 1 because the value of \(N\) has decreased. |
\(4.87 \times 10^3\) |
| b |
Converting the exponents to the same value gives either \((6.923 \times 10^{-3}) − (0.8756 \times 10^{-3}) = (6.923 − 0.8756) \times 10^{−3}\) or \((69.23 \times 10^{-4}) − (8.756 \times 10^{-4}) = (69.23 − 8.756) \times 10^{−4} = 60.474 \times 10^{−4}\). In converting 60.474 × 10-4 to scientific notation, \(n\) has become more positive by 1 because the value of \(N\) has decreased. |
\(6.047 \times 10^{−3}\) |
Scientific Notation: Multiplication and Division
When multiplying numbers expressed in scientific notation, we multiply the values of \(N\) and add together the values of \(n\). Conversely, when dividing, we divide \(N\) in the dividend (the number being divided) by \(N\) in the divisor (the number by which we are dividing) and then subtract n in the divisor from n in the dividend. In contrast to addition and subtraction, the exponents do not have to be the same in multiplication and division. Examples of problems involving multiplication and division are shown in Example \(\PageIndex{3}\).
Perform the appropriate operation and express your answer in scientific notation.
- \([ (6.022 \times 10^{23})(6.42 \times 10^{−2}) \nonumber\)
- \( \dfrac{ 1.67 \times 10^{-24} }{ 9.12 \times 10 ^{-28} } \nonumber \)
- \( \dfrac{ (6.63 \times 10^{−34})(6.0 \times 10) }{ 8.52 \times 10^{−2}} \nonumber \)
Solution
| Explanation | Answer | |
|---|---|---|
| a |
In multiplication, we add the exponents: \[(6.022 \times 10^{23})(6.42 \times 10^{−2})= (6.022)(6.42) \times 10^{[23 + (−2)]} = 38.7 \times 10^{21} \nonumber\] In converting \(38.7 \times 10^{21}\) to scientific notation, \(n\) has become more positive by 1 because the value of \(N\) has decreased. |
\(3.87 \times 10^{22}\) |
| b |
In division, we subtract the exponents: \[{1.67 \times 10^{−24} \over 9.12 \times 10^{−28}} = {1.67 \over 9.12} \times 10^{[−24 − (−28)]} = 0.183 \times 10^4 \nonumber\] In converting \(0.183 \times 10^4\) to scientific notation, \(n\) has become more negative by 1 because the value of \(N\) has increased. |
\( 1.83 \times 10^3\) |
| c |
This problem has both multiplication and division: \[ {(6.63 \times 10^{−34})(6.0 \times 10) \over (8.52 \times 10^{−2})} = {39.78 \over 8.52} \times 10^{[−34 + 1 − (−2)]} \nonumber \] |
\( 4.7\times 10^{-31}\) |
Key Takeaways
- Identifying a quantity properly requires both a number and a unit.
- Metric prefixes derive from Latin or Greek terms. The prefixes are used to make the units manageable.
- The SI system is based on multiples of ten. There are seven basic units in the SI system. Five of these units are commonly used in chemistry.

