16.3: Fats and Other Lipids
- Page ID
- 152240
- Recognize the structures of common fatty acids.
- Describe the structure of fats and oils and classify them as saturated, monounsaturated, or polyunsaturated.
Fats and oils, found in many of the foods we eat, belong to a class of biomolecules known as lipids. Gram for gram, they pack more than twice the caloric content of carbohydrates: the oxidation of fats and oils supplies about 9 kcal of energy for every gram oxidized, whereas the oxidation of carbohydrates supplies only 4 kcal/g. Although the high caloric content of fats may be bad news for the dieter, it says something about the efficiency of nature’s designs. Our bodies use carbohydrates, primarily in the form of glucose, for our immediate energy needs. Our capacity for storing carbohydrates for later use is limited to tucking away a bit of glycogen in the liver or in muscle tissue. We store our reserve energy in lipid form, which requires far less space than the same amount of energy stored in carbohydrate form. Lipids have other biological functions besides energy storage. They are a major component of the membranes of the 10 trillion cells in our bodies. They serve as protective padding and insulation for vital organs. Furthermore, without lipids in our diets, we would be deficient in the fat-soluble vitamins A, D, E, and K.
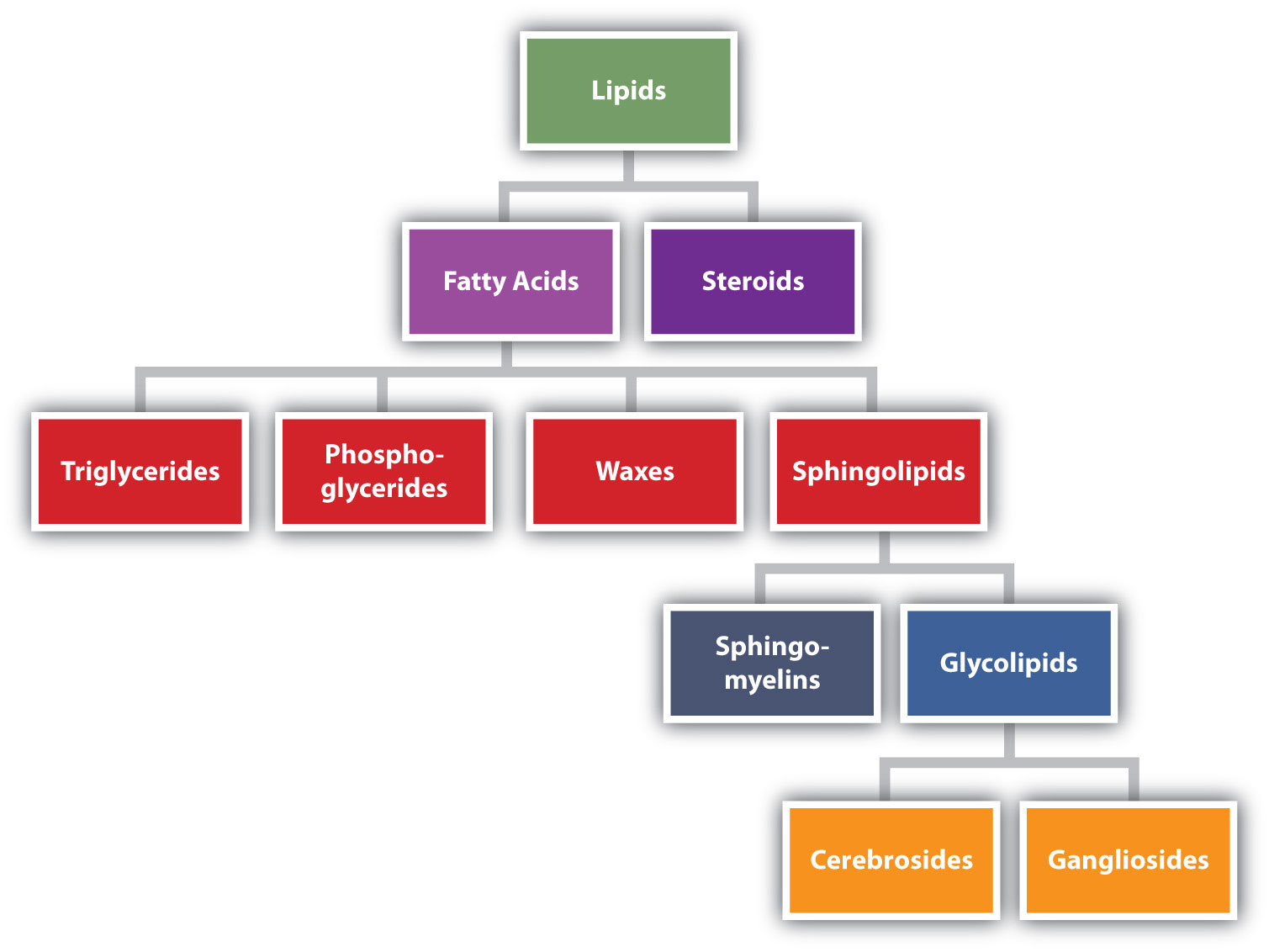
Lipids are not defined by the presence of specific functional groups, as carbohydrates are, but by a physical property—solubility. Compounds isolated from body tissues are classified as lipids if they are more soluble in organic solvents, such as dichloromethane, than in water. By this criterion, the lipid category includes not only fats and oils, which are esters of the trihydroxy alcohol glycerol and fatty acids, but also compounds that incorporate functional groups derived from phosphoric acid, carbohydrates, or amino alcohols, as well as steroid compounds such as cholesterol (Figure \(\PageIndex{1}\) presents one scheme for classifying the various kinds of lipids). We will discuss the various kinds of lipids by considering one subclass at a time and pointing out structural similarities and differences as we go.
Fatty Acids
Fatty acids are carboxylic acids that are structural components of fats, oils, and all other categories of lipids, except steroids. More than 70 have been identified in nature. They usually contain an even number of carbon atoms (typically 12–20), are generally unbranched, and can be classified by the presence and number of carbon-to-carbon double bonds. Thus, saturated fatty acids contain no carbon-to-carbon double bonds, monounsaturated fatty acids contain one carbon-to-carbon double bond, and polyunsaturated fatty acids contain two or more carbon-to-carbon double bonds.

| Name | Abbreviated Structural Formula | Condensed Structural Formula | Melting Point (°C) | Source |
|---|---|---|---|---|
| lauric acid | C11H23COOH | CH3(CH2)10COOH | 44 | palm kernel oil |
| myristic acid | C13H27COOH | CH3(CH2)12COOH | 58 | oil of nutmeg |
| palmitic acid | C15H31COOH | CH3(CH2)14COOH | 63 | palm oil |
| palmitoleic acid | C15H29COOH | CH3(CH2)5CH=CH(CH2)7COOH | 0.5 | macadamia oil |
| stearic acid | C17H35COOH | CH3(CH2)16COOH | 70 | cocoa butter |
| oleic acid | C17H33COOH | CH3(CH2)7CH=CH(CH2)7COOH | 16 | olive oil |
| linoleic acid | C17H31COOH | CH3(CH2)3(CH2CH=CH)2(CH2)7COOH | −5 | canola oil |
| α-linolenic acid | C17H29COOH | CH3(CH2CH=CH)3(CH2)7COOH | −11 | flaxseed |
| arachidonic acid | C19H31COOH | CH3(CH2)4(CH2CH=CH)4(CH2)2COOH | −50 | liver |
Two polyunsaturated fatty acids—linoleic and α-linolenic acids—are termed essential fatty acids because humans must obtain them from their diets. Both substances are required for normal growth and development, but the human body does not synthesize them. The body uses linoleic acid to synthesize many of the other unsaturated fatty acids, such as arachidonic acid, a precursor for the synthesis of prostaglandins. In addition, the essential fatty acids are necessary for the efficient transport and metabolism of cholesterol. The average daily diet should contain about 4–6 g of the essential fatty acids.
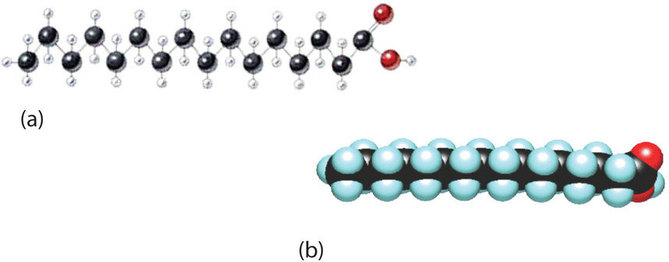
Fats and oils are the most abundant lipids in nature. They provide energy for living organisms, insulate body organs, and transport fat-soluble vitamins through the blood. Although we often draw the carbon atoms in a straight line, they actually have more of a zigzag configuration (Figure \(\PageIndex{2a}\)). Viewed as a whole, however, the saturated fatty acid molecule is relatively straight (Figure \(\PageIndex{2b}\)). Such molecules pack closely together into a crystal lattice, maximizing the strength of dispersion forces and causing fatty acids and the fats derived from them to have relatively high melting points. In contrast, each cis carbon-to-carbon double bond in an unsaturated fatty acid produces a pronounced bend in the molecule, so that these molecules do not stack neatly. As a result, the intermolecular attractions of unsaturated fatty acids (and unsaturated fats) are weaker, causing these substances to have lower melting points. Most are liquids at room temperature.
Structures of Fats and Oils
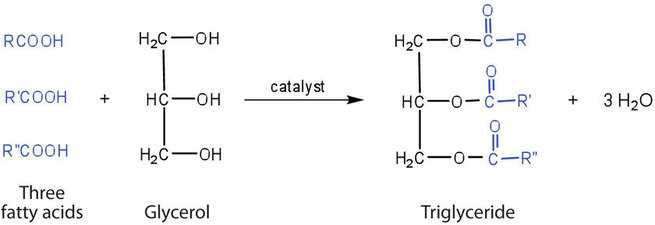
Fats and oils are called triglycerides (or triacylcylgerols) because they are esters composed of three fatty acid units joined to glycerol, a trihydroxy alcohol:
If all three OH groups on the glycerol molecule are esterified with the same fatty acid, the resulting ester is called a simple triglyceride. Although simple triglycerides have been synthesized in the laboratory, they rarely occur in nature. Instead, a typical triglyceride obtained from naturally occurring fats and oils contains two or three different fatty acid components and is thus termed a mixed triglyceride.
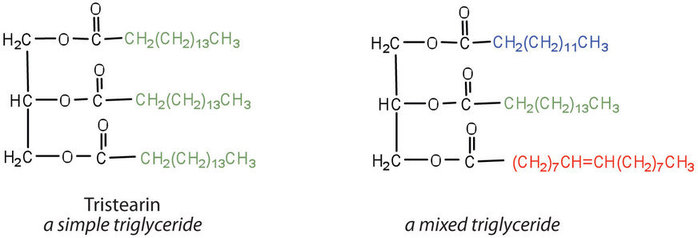
A triglyceride is called a fat if it is a solid at 25°C; it is called an oil if it is a liquid at that temperature. These differences in melting points reflect differences in the degree of unsaturation and number of carbon atoms in the constituent fatty acids. Triglycerides obtained from animal sources are usually solids, while those of plant origin are generally oils. Therefore, we commonly speak of animal fats and vegetable oils.
No single formula can be written to represent the naturally occurring fats and oils because they are highly complex mixtures of triglycerides in which many different fatty acids are represented. Table \(\PageIndex{2}\) shows the fatty acid compositions of some common fats and oils. The composition of any given fat or oil can vary depending on the plant or animal species it comes from as well as on dietetic and climatic factors. To cite just one example, lard from corn-fed hogs is more highly saturated than lard from peanut-fed hogs. Palmitic acid is the most abundant of the saturated fatty acids, while oleic acid is the most abundant unsaturated fatty acid.
| Lauric | Myristic | Palmitic | Stearic | Oleic | Linoleic | Linolenic | |
|---|---|---|---|---|---|---|---|
| Fats | |||||||
| butter (cow) | 3 | 11 | 27 | 12 | 29 | 2 | 1 |
| tallow | 3 | 24 | 19 | 43 | 3 | 1 | |
| lard | 2 | 26 | 14 | 44 | 10 | ||
| Oils | |||||||
| canola oil | 4 | 2 | 62 | 22 | 10 | ||
| coconut oil† | 47 | 18 | 9 | 3 | 6 | 2 | |
| corn oil | 11 | 2 | 28 | 58 | 1 | ||
| olive oil | 13 | 3 | 71 | 10 | 1 | ||
| peanut oil | 11 | 2 | 48 | 32 | |||
| soybean oil | 11 | 4 | 24 | 54 | 7 | ||
| *Totals less than 100% indicate the presence of fatty acids with fewer than 12 carbon atoms or more than 18 carbon atoms. | |||||||
| †Coconut oil is highly saturated. It contains an unusually high percentage of the low-melting C8, C10, and C12 saturated fatty acids. | |||||||
Terms such as saturated fat or unsaturated oil are often used to describe the fats or oils obtained from foods. Saturated fats contain a high proportion of saturated fatty acids, while unsaturated oils contain a high proportion of unsaturated fatty acids. The high consumption of saturated fats is a factor, along with the high consumption of cholesterol, in increased risks of heart disease.
Physical Properties of Fats and Oils
Contrary to what you might expect, pure fats and oils are colorless, odorless, and tasteless. The characteristic colors, odors, and flavors that we associate with some of them are imparted by foreign substances that are lipid soluble and have been absorbed by these lipids. For example, the yellow color of butter is due to the presence of the pigment carotene; the taste of butter comes from two compounds—diacetyl and 3-hydroxy-2-butanone—produced by bacteria in the ripening cream from which the butter is made.
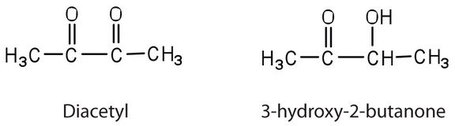
Fats and oils are lighter than water, having densities of about 0.8 g/cm3. They are poor conductors of heat and electricity and therefore serve as excellent insulators for the body, slowing the loss of heat through the skin.
Chemical Reactions of Fats and Oils
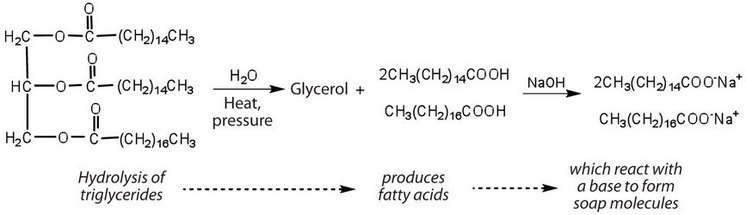
Fats and oils can participate in a variety of chemical reactions—for example, because triglycerides are esters, they can be hydrolyzed in the presence of an acid, a base, or specific enzymes known as lipases. The hydrolysis of fats and oils in the presence of a base is used to make soap and is called saponification. Today most soaps are prepared through the hydrolysis of triglycerides (often from tallow, coconut oil, or both) using water under high pressure and temperature [700 lb/in2 (∼50 atm or 5,000 kPa) and 200°C]. Sodium carbonate or sodium hydroxide is then used to convert the fatty acids to their sodium salts (soap molecules):
Iodine Value
The iodine value (or iodine adsorption value or iodine number or iodine index, commonly abbreviated as IV) in chemistry is the mass of iodine in grams that is consumed by 100 grams of a chemical substance. Iodine numbers are often used to determine the amount of unsaturation in fats, oils and waxes. In fatty acids, unsaturation occurs mainly as double bonds which are very reactive towards halogens, the iodine in this case. Thus, the higher the iodine value, the more unsaturations are present in the fat. It can be seen from the table that coconut oil is very saturated, which means it is good for making soap. On the other hand, linseed oil is highly unsaturated, which makes it a drying oil, well suited for making oil paints.
| Fat | Iodine Value (g/100g) |
| Beef tallow | 42-48 |
| Butter | 25-42 |
| Canola Oil | 110-126 |
| Coconut Oil | 6-11 |
| Fish oil | 190-205 |
| Linseed Oil | 170-204 |
| Olive Oil | 75-94 |
| Safflower Oil | 135-150 |
| Walnut Oil | 132-162 |
Ordinary soap is a mixture of the sodium salts of various fatty acids, produced in one of the oldest organic syntheses practiced by humans (second only to the fermentation of sugars to produce ethyl alcohol). Both the Phoenicians (600 BCE) and the Romans made soap from animal fat and wood ash. Even so, the widespread production of soap 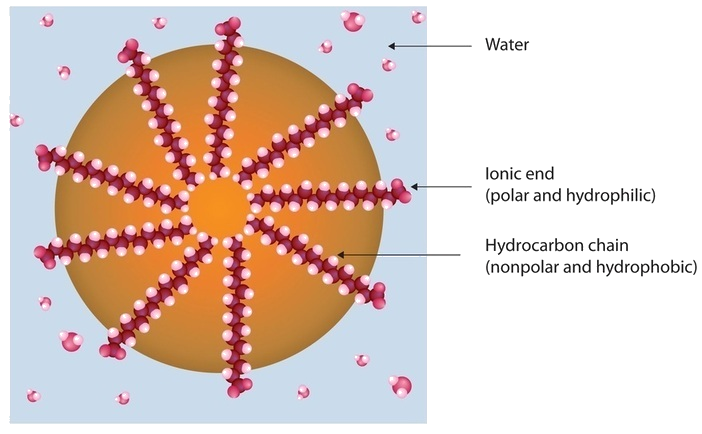 did not begin until the 1700s. Soap was traditionally made by treating molten lard or tallow with a slight excess of alkali in large open vats. The mixture was heated, and steam was bubbled through it. After saponification was completed, the soap was precipitated from the mixture by the addition of sodium chloride (NaCl), removed by filtration, and washed several times with water. It was then dissolved in water and reprecipitated by the addition of more NaCl. The glycerol produced in the reaction was also recovered from the aqueous wash solutions.
did not begin until the 1700s. Soap was traditionally made by treating molten lard or tallow with a slight excess of alkali in large open vats. The mixture was heated, and steam was bubbled through it. After saponification was completed, the soap was precipitated from the mixture by the addition of sodium chloride (NaCl), removed by filtration, and washed several times with water. It was then dissolved in water and reprecipitated by the addition of more NaCl. The glycerol produced in the reaction was also recovered from the aqueous wash solutions.
Pumice or sand is added to produce scouring soap, while ingredients such as perfumes or dyes are added to produce fragrant, colored soaps. Blowing air through molten soap produces a floating soap. Soft soaps, made with potassium salts, are more expensive but produce a finer lather and are more soluble. They are used in liquid soaps, shampoos, and shaving creams.
Dirt and grime usually adhere to skin, clothing, and other surfaces by combining with body oils, cooking fats, lubricating greases, and similar substances that act like glues. Because these substances are not miscible in water, washing with water alone does little to remove them. Soap removes them, however, because soap molecules have a dual nature. One end, called the head, carries an ionic charge (a carboxylate anion) and therefore dissolves in water; the other end, the tail, has a hydrocarbon structure and dissolves in oils. The hydrocarbon tails dissolve in the soil; the ionic heads remain in the aqueous phase, and the soap breaks the oil into tiny soap-enclosed droplets called micelles, which disperse throughout the solution. The droplets repel each other because of their charged surfaces and do not coalesce. With the oil no longer “gluing” the dirt to the soiled surface (skin, cloth, dish), the soap-enclosed dirt can easily be rinsed away.
Summary
- Fatty acids are carboxylic acids that are the structural components of many lipids.
- Saturated fatty acids contain no carbon-to-carbon double bonds, monounsaturated fatty acids contain one carbon-to-carbon double bond, and polyunsaturated fatty acids contain two or more carbon-to-carbon double bonds.
- Fats and oils are composed of molecules known as triglycerides, which are esters composed of three fatty acid units linked to glycerol.
- Fats and oils can participate in a variety of chemical reactions—for example, because triglycerides are esters, they can be hydrolyzed in the presence of an acid, a base, or specific enzymes known as lipases.
- The hydrolysis of fats and oils in the presence of a base is used to make soap and is called saponification.
- Animal fats and oils which are made up mostly of saturated fatty acids have low iodine values (numbers), while those oils with high degree of unsaturation (with more C=C bonds) have high iodine values.
Contributors and Attributions
- Libretext: The Basics od GOB Chemistry (Ball et al.)
- Wikipedia

