10.1: Polymerization - Making Big Ones Out of Little Ones
- Page ID
- 152196
- Define the terms monomer and polymer.
- Know the different types of natural polymers.
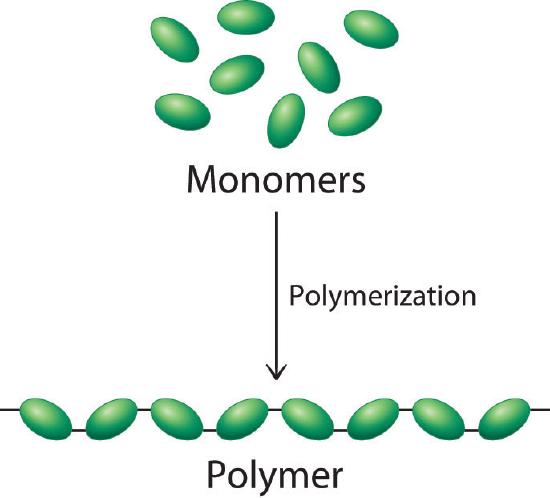
A polymer is a large molecule, or macromolecule, composed of many repeated subunits.The term "polymer" derives from the Greek word polus (meaning "many, much") and meros (meaning "part"), and refers to a molecule whose structure is composed of multiple repeating units, from which originates a characteristic of high relative molecular mass and attendant properties. As shown schematically in Figure \(\PageIndex{1}\).
Due to their broad range of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass relative to small molecule compounds produces unique physical properties, including toughness, viscoelasticity, and a tendency to form glasses and semicrystalline structures rather than crystals. The terms polymer and resin are often synonymous with plastic.
Natural Polymers
Some very important biological materials are polymers. Of the three major food groups, polymers are represented in two: proteins and carbohydrates. Proteins are polymers of amino acids, which are monomers that have an amine functional group and a carboxylic acid functional group. Proteins play a crucial role in living organisms.
Linking hundreds of glucose molecules together makes a relatively common material known as starch:
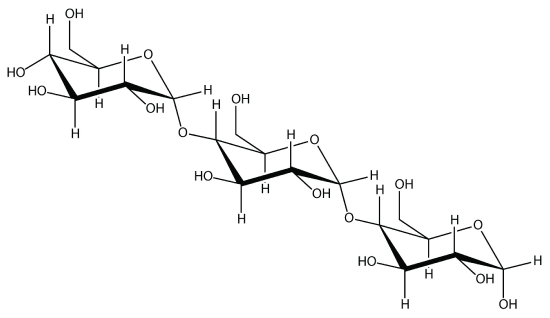
Starch is an important source of energy in the human diet. Note how individual glucose units are joined together. They can also be joined together in another way, like this:
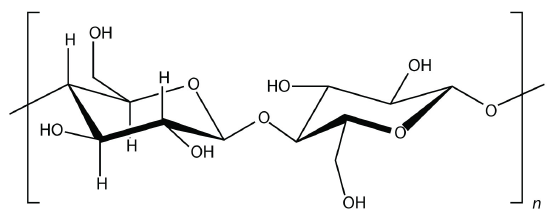
This polymer is known as cellulose. Cellulose is a major component in the cell walls of plants. Curiously, despite the similarity in the building blocks, some animals (such as humans) cannot digest cellulose; those animals that can digest cellulose typically rely on symbiotic bacteria in the digestive tract for the actual digestion. Animals do not have the proper enzymes to break apart the glucose units in cellulose, so it passes through the digestive tract and is considered dietary fiber.
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are also polymers, composed of long, three-part chains consisting of phosphate groups, sugars with 5 C atoms (ribose or deoxyribose), and N-containing rings referred to as bases. Each combination of the three parts is called a nucleotide; DNA and RNA are essentially polymers of nucleotides that have rather complicated but intriguing structures (Figure \(\PageIndex{2}\) - Nucleotides). DNA is the fundamental material in chromosomes and is directly responsible for heredity, while RNA is an essential substance in protein synthesis.

The DNA in our cells is a polymer of nucleotides, each of which is composed of a phosphate group, a sugar, and a N-containing base
The above mentioned biopolymers (polymers produced by living organisms) are discussed further in Chapter 16.
Celluloid: Billiard Balls
Celluloids are a class of compounds created from nitrocellulose (partially nitrated cellulose) and camphor, with added dyes and other agents. Generally considered the first thermoplastic, it was first created as Parkesinein (by Alexander Parkes of Birmingham England) in 1856 and as Xylonite in 1869. In the 1860s, an American, John Wesley Hyatt, acquired Parkes's patent and began experimenting with cellulose nitrate with the intention of manufacturing billiard balls, which until that time were made from ivory. In the 1870s the modified plastic was registered as "celluloid".
The main use was in movie and photography film industries, which used only celluloid film stock prior to the adoption of acetate safety film in the 1950s. Celluloid is highly flammable, difficult and expensive to produce and no longer widely used; its most common uses today are in table tennis balls, musical instruments, and guitar picks.
Bakelite (sometimes spelled Baekelite) or polyoxybenzylmethylenglycolanhydride was the first plastic made from synthetic components. It is a thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. It was developed by the Belgian-American chemist Leo Baekeland in Yonkers, New York, in 1907.
Bakelite was patented on December 7, 1909. The creation of a synthetic plastic was revolutionary for its electrical non conductivity and heat-resistant properties in electrical insulators, radio and telephone casings and such diverse products as kitchenware, jewelry, pipe stems, children's toys, and firearms.
Video \(\PageIndex{2}\) Polymers Crash Course
Summary
- Polymers are giant molecules that consist of long chains of units called monomers connected by covalent bonds.
- Polymerization is the process of linking monomers together to form a polymer.
- Plastic is the general term for polymers made from synthetic materials.
- Several important biological polymers include proteins, starch, cellulose, DNA and RNA.
Contributors and Attributions
- Joshua Halpern, Scott Sinex and Scott Johnson
- TextMap: Beginning Chemistry (Ball et al.)
- Wikipedia

