9.9: Properties of Solutions
- Page ID
- 86670
- To describe how the properties of solutions differ from those of pure solvents.
Solutions are likely to have properties similar to those of their major component—usually the solvent. However, some solution properties differ significantly from those of the solvent. Here, we will focus on liquid solutions that have a solid solute, but many of the effects we will discuss in this section are applicable to all solutions.
Colligative Properties
Solutes affect some properties of solutions that depend only on the concentration of the dissolved particles. These properties are called colligative properties. Four important colligative properties that we will examine are vapor pressure depression, boiling point elevation, freezing point depression, and osmotic pressure.
Molecular compounds separate into individual molecules when they are dissolved, so for every 1 mol of molecules dissolved, we get 1 mol of particles. In contrast, ionic compounds separate into their constituent ions when they dissolve, so 1 mol of an ionic compound will produce more than 1 mol of dissolved particles. For example, every mole of NaCl that dissolves yields 1 mol of Na+ ions and 1 mol of Cl− ions, for a total of 2 mol of particles in solution. Thus, the effect on a solution’s properties by dissolving NaCl may be twice as large as the effect of dissolving the same amount of moles of glucose (C6H12O6).
Vapor Pressure Depression
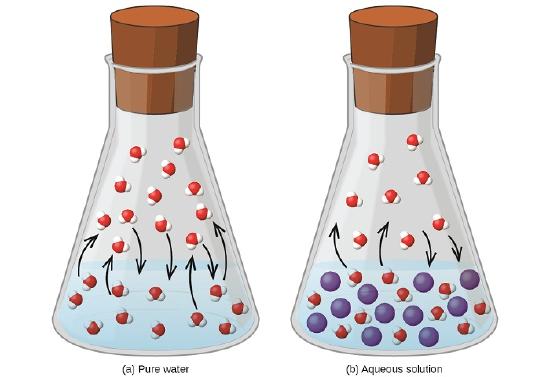
All liquids evaporate. In fact, given enough volume, a liquid will turn completely into a vapor. If enough volume is not present, a liquid will evaporate only to the point where the rate of evaporation equals the rate of vapor condensing back into a liquid. The pressure of the vapor at this point is called the vapor pressure of the liquid.
The presence of a dissolved solid lowers the characteristic vapor pressure of a liquid so that it evaporates more slowly. (The exceptions to this statement are if the solute itself is a liquid or a gas, in which case the solute will also contribute something to the evaporation process. We will not discuss such solutions here.) This property is called vapor pressure depression and is depicted in Figure \(\PageIndex{1}\).
Boiling Point Elevation
A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed to reach the boiling point. This phenomenon is called boiling point elevation. For every mole of particles dissolved in a liter of water, the boiling point of water increases by about 0.51°C.
\[\Delta T_{boiling} = mol\ particles \times 0.51°C\nonumber \]
The addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100°C to 100.51°C, but the addition of one mole of NaCl in one liter of water will raise the boiling point by 2 x 0.51°C = 1.02°C. Furthermore, the addition of one mole of \(\ce{CaCl2}\) in one liter of water will raise the boiling point by 3 x 0.51°C = 1.53°C.
When cooking dried pasta, many recipes call for salting the water before cooking the pasta. Some argue—with colligative properties on their side—that adding salt to the water raises the boiling point, thus cooking the pasta faster. Is there any truth to this?

In order to increase the boiling temperature of 4 L of water by 1.0°C, with the presumption that dried pasta cooks noticeably faster at 101°C than at 100°C (although a 1° difference may make only a negligible change in cooking times), over 1 lb of salt (~1 cup) would be needed. In your experience, do you add almost a cup of salt to a pot of water to make pasta? Certainly not! A few pinches, perhaps one-fourth of a teaspoon, but not almost a cup! It is obvious that the little amount of salt that most people add to their pasta water is not going to significantly raise the boiling point of the water.
So why do people add some salt to boiling water? There are several possible reasons, the most obvious of which is taste: adding salt adds a little bit of salt flavor to the pasta. It cannot be much because most of the salt remains in the water, not in the cooked pasta. However, it may be enough to detect with our taste buds. The other obvious reason is habit; recipes tell us to add salt, so we do, even if there is little scientific or culinary reason to do so.
Freezing Point Depression
The presence of solute particles has the opposite effect on the freezing point of a solution. When a solution freezes, only the solvent particles come together to form a solid phase, and the presence of solute particles interferes with that process. Therefore, for the liquid solvent to freeze, more energy must be removed from the solution, which lowers the temperature. Thus, solutions have lower freezing points than pure solvents do. This phenomenon is called freezing point depression. For every mole of particles in a liter of water, the freezing point decreases by about 1.86°C.
\[\Delta T_{freezing} = mol\ particles \times -1.86°C\nonumber \]
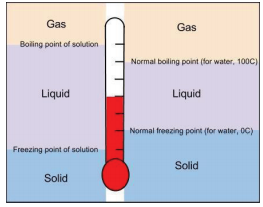
Both boiling point elevation and freezing point depression have practical uses. For example, solutions of water and ethylene glycol (C2H6O2) are used as coolants in automobile engines because the boiling point of such a solution is greater than 100°C, the normal boiling point of water. In winter, salts like NaCl and \(\ce{CaCl_2}\) are sprinkled on the ground to melt ice or keep ice from forming on roads and sidewalks (Figure \(\PageIndex{4}\)). This is because the solution made by dissolving sodium chloride or calcium chloride in water has a lower freezing point than pure water, so the formation of ice is inhibited.

Which solution’s freezing point deviates more from that of pure water—a 1 M solution of NaCl or a 1 M solution of \(\ce{CaCl_2}\)?
Solution
Colligative properties depend on the number of dissolved particles, so the solution with the greater number of particles in solution will show the greatest deviation. When NaCl dissolves, it separates into two ions, Na+ and Cl−. But when \(\ce{CaCl2}\) dissolves, it separates into three ions—one Ca2+ ion and two Cl− ions. Thus, mole for mole, \(\ce{CaCl2}\) will have 50% more impact on freezing point depression than NaCl.
Which solution’s boiling point deviates more from that of pure water—a 1 M solution of \(\ce{CaCl2}\) or a 1 M solution of MgSO4?
- Answer
-
\(\ce{CaCl2}\)
Estimate the boiling point of 0.2 M \(\ce{CaCl2}\) solution.
Solution
The boiling point increases 0.51°C for every mole of solute per liter of water. For this estimation, let's assume that 1 liter of solution is roughly the same volume as 1 liter of water. A 0.2 M \(\ce{CaCl_2}\) solution contains 0.2 moles of \(\ce{CaCl_2}\) solution formula units per liter of solution. Each \(\ce{CaCl_2}\) unit separates into three ions.
\[\mathrm{0.2\: mol\: CaCl_2\times\dfrac{3\: mol\: ions}{1\: mol\: CaCl_2}\times\dfrac{0.51° C}{1\: mol\: ion}=0.306° C} \nonumber \]
The normal boiling point of water is 100°C, so the boiling point of the solution is raised to 100.31°C.
Estimate the freezing point of 0.3 M \(\ce{CaCl_2}\) solution.
- Answer
-
–1.7°C

