21.4: Arrhenius Bases
- Page ID
- 53933
Sodium hydroxide is a versatile chemical. It can be used for such mundane purposes as cleaning clogged drains. Several commercial preparations contain sodium hydroxide for this purpose. It also has a number of applications in the food processing field. Ice cream is thickened using \(\ce{NaOH}\). If olives are soaked in a solution containing sodium hydroxide and other chemicals, the olives will turn black. Soft pretzels are made chewy by the application of sodium hydroxide to the food. This compound has been widely used in the synthesis of plastics, for etching aluminum, for paint removal, and is employed in the dehorning of cattle.
Arrhenius Bases
An Arrhenius base is a compound that ionizes to yield hydroxide ions \(\left( \ce{OH^-} \right)\) in aqueous solution. The table below lists several of the more common bases.
| Base Name | Formula |
|---|---|
| Sodium hydroxide | \(\ce{NaOH}\) |
| Potassium hydroxide | \(\ce{KOH}\) |
| Magnesium hydroxide | \(\ce{Mg(OH)_2}\) |
| Calcium hydroxide | \(\ce{Ca(OH)_2}\) |
All of the bases listed in the table are solids at room temperature. Upon dissolving in water, each dissociates into a metal cation and the hydroxide ion.
\[\ce{NaOH} \left( s \right) \rightarrow \ce{Na^+} \left( aq \right) + \ce{OH^-} \left( aq \right)\nonumber \]
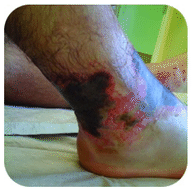
Sodium hydroxide is a very caustic substance also known as lye. Lye is used as a rigorous cleaner and is an ingredient in the manufacture of soaps. Care must be taken with strong bases like sodium hydroxide, as exposure can lead to severe burns (see figure below).
Sodium belongs to the group of elements called the alkali metals. An alkaline solution is another name for a solution that is basic. All alkali metals react readily with water to produce the metal hydroxide and hydrogen gas. The resulting solutions are basic.
\[2 \ce{K} \left( s \right) + 2 \ce{H_2O} \left( l \right) \rightarrow 2 \ce{KOH} \left( aq \right) + \ce{H_2} \left( g \right)\nonumber \]
Bases that consist of an alkali metal cation and the hydroxide anion are all very soluble in water. Compounds of the Group 2 metals (the alkaline earth metals) are also basic. However, these compounds are generally not as soluble in water. Therefore, the dissociation reactions for these compounds are shown as equilibrium reactions:
\[\ce{Mg(OH)_2} \left( s \right) \overset{\ce{H_2O}}{\rightleftharpoons} \ce{Mg^{2+}} \left( aq \right) + 2 \ce{OH^-} \left( aq \right)\nonumber \]
These relatively insoluble hydroxides were some of the compounds discussed in the context of the solubility product constant \(\left( K_\text{sp} \right)\). The solubility of magnesium hydroxide is \(0.0084 \: \text{g}\) per liter of water at \(25^\text{o} \text{C}\). Because of its low solubility, magnesium hydroxide is not as dangerous as sodium hydroxide. In fact, magnesium hydroxide is the active ingredient in a product called milk of magnesia, which is used as an antacid or a mild laxative.
Summary
- Arrhenius base is a compound that ionizes to yield hydroxide ions \(\left( \ce{OH^-} \right)\) in aqueous solution.
- Examples of Arrhenius bases are given.

