4.1: The Art of Deduction- Stable Electron Configurations
- Page ID
- 152154
- Define the octet rule.
The octet rule is a chemical rule of thumb that reflects observation that elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.
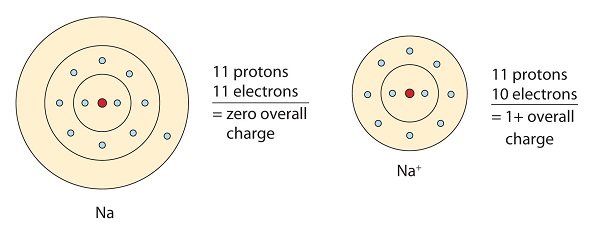
Referring to the octet rule, atoms attempt to get a noble gas electron configuration, which is eight valence electrons. Metals have few electrons in their outer-most orbitals. By losing those electrons, these metals can achieve noble gas configuration and satisfy the octet rule. Sodium has one valence electron, so giving it up would result in the same electron configuration as neon. Due to the loss of electron, a positively charged cation (Na+) called sodium ion is formed.
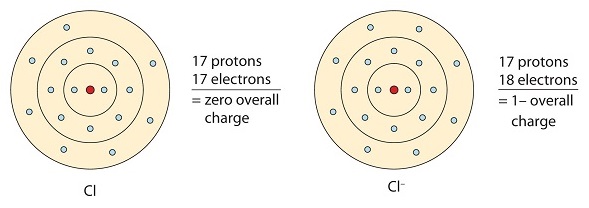
Similarly, nonmetals that have close to 8 electrons in their valence shells tend to readily accept electrons to achieve noble gas configuration. Chlorine has seven valence electrons, so if it takes one it will have eight (an octet). Chlorine has the electron configuration of argon when it gains an electron. The resulting ion (Cl-) is called chloride ion.
The octet rule could have been satisfied if chlorine gave up all seven of its valence electrons and sodium took them. In that case, both would have the electron configurations of noble gasses, with a full valence shell. However, their charges would be much higher. It would be Na7- and Cl7+, which is much less stable than Na+ and Cl-. Atoms are more stable when they have no charge, or a small charge.
Summary
The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell.
Contributor
Organic Chemistry Supplemental_Modules_(Organic_Chemistry)/Fundamentals/Ionic_and_Covalent_Bonds

