2.6: Atoms and Molecules- Real and Relevant
- Page ID
- 152145
- Know the difference between an atom and a molecule.
An atom is the smallest unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of either neutral (un-ionized), or ionized atoms. Atoms are extremely small; typical sizes are around 100 picometers (1×10−10 m, a ten-millionth of a millimeter, or 1/254,000,000 of an inch). They are small enough that attempting to predict their behavior using classical physics—as if they were billiard balls, for example—gives noticeably incorrect predictions due to quantum effects. Current atomic models have incorporated quantum mechanics to better explain and predict this behavior.
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons; only the most common variety (isotope) of hydrogen has no neutrons.
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge. However, in quantum physics, organic chemistry, and biochemistry, the term molecule is often used less strictly—as it is also applied to polyatomic ions.
In the kinetic theory of gases, the term molecule is often used for any gas, regardless of its composition. According to this definition, noble gas atoms are considered molecules because they are monatomic molecules.
A molecule may be homonuclear—that is, it consists of atoms of one chemical element, as with oxygen (O2). A heteronuclear molecule is a chemical compound composed of more than one element, as with water (H2O). Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not considered single molecules.
Molecules as components of matter are common in organic substances (and therefore biochemistry). They also make up most of the oceans and atmosphere. However, the majority of familiar solid substances on Earth—including most of the minerals that make up the crust, mantle, and core of the Earth—contain many chemical bonds, but are not made of identifiable molecules. Also, no typical molecule can be defined for ionic crystals (salts) and covalent crystals (network solids), although these are often composed of repeating unit cells that extend either in a plane (such as in graphene) or three-dimensionally (such as in diamond, quartz, or sodium chloride). The theme of repeated unit-cellular-structure also holds for most condensed phases with metallic bonding, which means that solid metals are also not made of molecules. In glasses (solids that exist in a vitreous disordered state), atoms may also be held together by chemical bonds with no presence of any definable molecule, nor any of the regularity of repeating units that characterizes crystals.
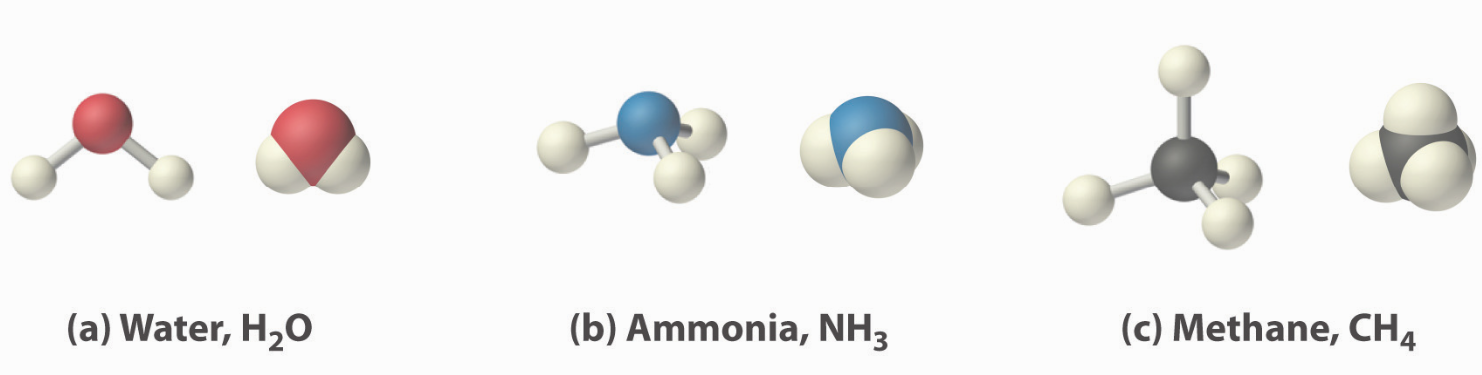
Three common molecules shown below.
Video \(\PageIndex{1}\): The difference between an atom and a molecule.
Summary
- An atom is the smallest unit of matter that has the properties of a chemical element
- A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.
Contributors and Attributions
- Wikipedia
Henry Agnew (UC Davis)

