2.2: Scientific Laws - Conservation of Mass and Definite Proportions
- Page ID
- 152141
- Explain the law of conservation of mass.
- Explain the law of definite proportions.
The Law of Conservation of Mass
It may seem as though burning destroys matter, but the same amount, or mass, of matter still exists after a campfire as before. Figure \(\PageIndex{2}\) (below) shows that when wood burns, it combines with oxygen and changes not only to ashes, but also to carbon dioxide and water vapor. The gases float off into the air, leaving behind just the ashes. Suppose you had measured the mass of the wood before it burned and the mass of the ashes after it burned. Also assume that you had been able to measure the oxygen used by the fire and the gases produced by the fire. What would you find? The total mass of matter after the fire would be the same as the total mass of matter before the fire.
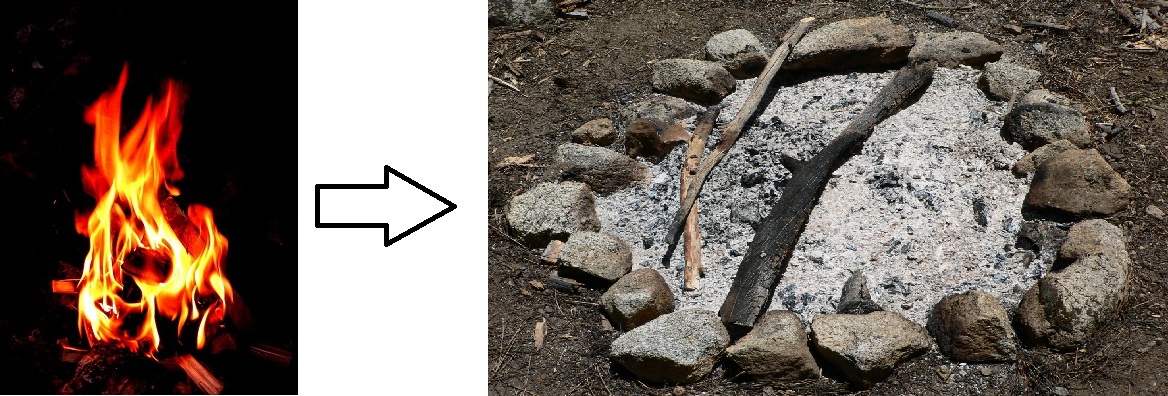
The law of conservation of mass was created in 1789 by French chemist Antoine Lavoisier. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example—in Figure \(\PageIndex{3}\)—when wood burns, the mass of the soot, ashes, and gases equals the original mass of the wood and the oxygen when it first reacted.
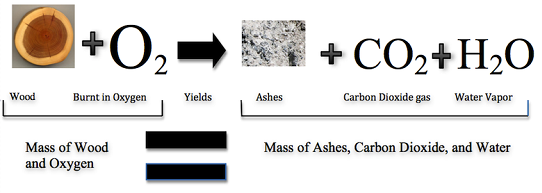
So, the mass of the product(s) equals the mass of the reactants. Reactants are two or more elements chemically interacting to make a new substance; product is the substance(s) formed as the result of a chemical reaction (Video \(\PageIndex{1}\)). Matter (and mass) may not be able to be created or destroyed, but can change forms to other substances like liquids, gases, solids, etc.
Video \(\PageIndex{1}\) This is a nice little demonstration showing the conservation of mass in action.
It's important to know the law well. If a 300 kg tree is burning down—when the process is complete—there are only ashes left, and all of them together weigh 10 kg. You may wonder where the other 290 kg went. The missing 290 kg was released into the atmosphere as smoke, so the only visible matter left is the 10 kg of ash. If you understand the law of conservation of mass, then you know that the other 290 kg had to go somewhere, because it had to equal the mass of the tree before it burnt down.
If heating 10.0 grams of calcium carbonate (CaCO3) produces 4.4 g of carbon dioxide (CO2) and 5.6 g of calcium oxide (CaO), show that these observations are in agreement with the law of conservation of mass.
Solution
\[\begin{align*} \text{Mass of the reactants} &= \text{Mass of the products} \\[4pt] 10.0\, \text{g of } \ce{CaCO3} &= 4.4 \,\text{g of }\ce{CO2} + 5.6\, \text{g of } \ce{ CaO} \\[4pt] 10.0\,\text{g of reactant} &= 10.0\, \text{g of products} \end{align*} \nonumber \]
Because the mass of the reactant is equal to the mass of the products, the observations are in agreement with the law of conservation of mass.
Potassium hydroxide (\(\ce{KOH}\)) readily reacts with carbon dioxide (\(\ce{CO2}\)) to produce Potassium carbonate (\(\ce{K2CO3}\)) and water (\(\ce{H2O}\)). How many grams of potassium carbonate are produced if 224.4 g of \(\ce{KOH}\) react with 88.0 g of \(\ce{CO2}\)? The reaction also produces 36.0 g of water.
- Answer
- 276.4 g of potassium carbonate
The Law of Definite Proportions
Joseph Proust (1754-1826) formulated the law of definite proportions (also called the Law of Constant Composition or Proust's Law). This law states that if a compound is broken down into its constituent elements, the masses of the constituents will always have the same proportions, regardless of the quantity or source of the original substance. Joseph Proust based this law primarily on his experiments with basic copper carbonate. The illustration below depicts this law in action.
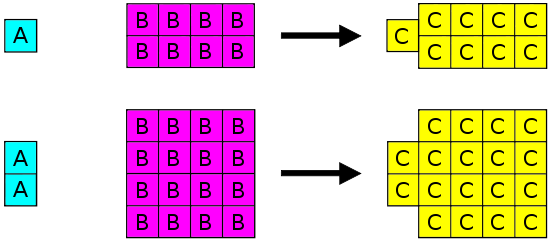
Law of Definite Proportions states that in a given type of chemical substance, the elements are always combined in the same proportions by mass.
The Law of Definite Proportions applies when elements are reacted together to form the same product. Therefore, while the Law of Definite Proportions can be used to compare two experiments in which hydrogen and oxygen react to form water, the Law of Definite Proportions can not be used to compare one experiment in which hydrogen and oxygen react to form water, and another experiment in which hydrogen and oxygen react to form hydrogen peroxide (peroxide is another material that can be made from hydrogen and oxygen).
Oxygen makes up 88.8% of the mass of any sample of pure water, while hydrogen makes up the remaining 11.2% of the mass. You can get water by melting ice or snow; or by condensing steam from a river, sea, pond, etc. It can be from different places: USA, UK, Australia, or anywhere. Water can be made by chemical reactions, such as burning hydrogen in oxygen.
However, if the water is pure, it will always consist of 88.8 % oxygen by mass and 11.2 % hydrogen by mass, irrespective of its source or method of preparation.
Video \(\PageIndex{2}\) Law of definite proportions.
Summary
- Burning and other changes in matter do not destroy matter.
- The mass of matter is always the same before and after the changes occur.
- The law of conservation of mass states that matter cannot be created or destroyed.
- The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass.
References
- Petrucci, Ralph, William Harwood, Geoffrey Herring, and Jeffry Madura. General Chemistry. 9th ed. Upper Saddle River, New Jersey: Pearson Prentince Hall, 2007.
- Moore, John. Chemistry for Dummies. John Wiley & Sons Inc, 2002.
- Asimov, Isaac. A Short History of Chemistry. , CT.: Greenwood Press, 1965.
- Patterson, Elizabeth C. John Dalton and the Atomic Theory. Garden City, NY: Doubleday, 1970.
- Myers, Richard. The Basics of Chemistry. Greenwood, 2003.
- Demtröder, Wolfgang. Atoms, Molecules and Photons: An Introduction to Atomic- Molecular- and Quantum Physics. 1st ed. Springer. 2002.
Contributors and Attributions
Binod Shrestha (University of Lorraine)
Henry Agnew (UC Davis)

