6.1: Early History of the Periodic Table
- Page ID
- 53031
When you go to the library to find a book, how do you locate it?
If it is a fiction book, you look by author since the fiction materials are filed by the author’s last name. If you are looking for a non-fiction publication, you look in a catalog (most likely on a computer these days). The book you are looking for will have a number by the title. This number refers to the Dewey Decimal system, developed by Melvil Dewey in 1876 and used in over 200,000 libraries throughout the world. Another system in wide use is the Library of Congress approach, developed in the late 1800s-early 1900s to organize the materials in the federal Library of Congress. This method is one of the most widely used ways to organize libraries in the world. Both approaches organize information so that people can easily find what they are looking for. Chemistry information also needs to be organized so we can see patterns of properties in elements.
Early Attempts to Organize Elements
By the year 1700, only a handful of elements had been identified and isolated. Several of these, such as copper and lead, had been known since ancient times. As scientific methods improved, the rate of discovery dramatically increased. With the ever-increasing number of elements, chemists recognized that there may be some kind of systematic way to organize the elements. The question was: how?
A logical way to begin grouping elements together was by their chemical properties. (In other words, putting elements in separate groups based on how they reacted with other elements.) In 1829, a German chemist, Johann Dobereiner (1780-1849), placed various groups of three elements into groups called triads. One such triad was lithium, sodium, and potassium. Triads were based on both physical, as well as chemical, properties. Dobereiner found that the atomic masses of these three elements, as well as other triads, formed a pattern. When the atomic masses of lithium and potassium were averaged together \(\left( \frac{\left( 6.94 + 39.10 \right)}{2} = 23.02 \right)\), it was approximately equal to the atomic mass of sodium (22.99). These three elements also displayed similar chemical reactions, such as vigorously reacting with the members of another triad: chlorine, bromine, and iodine. While Dobereiner's system would pave the way for future ideas, a limitation of the triad system was that not all of the known elements could be classified in this way.
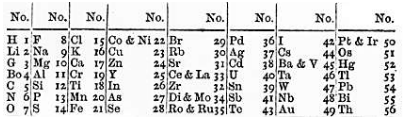
English chemist John Newlands (1838-1898) ordered the elements in increasing order of atomic mass and noticed that every eighth element exhibited similar properties. He called this relationship the "Law of Octaves". Unfortunately, there were some elements that were missing and the law did not seem to hold for elements that were heavier than calcium. Newlands' work was largely ignored and even ridiculed by the scientific community in his day. It was not until years later that another more extensive periodic table effort would gain much greater acceptance, and that the pioneering work of John Newlands would be appreciated.
Summary
- Johann Dobereiner organized elements into groups called triads.
- John Newlands proposed the "Law of Octaves" for organizing the elements.
Review
- List some elements known since ancient times?
- What properties were the basis of the triad system?
- Why did Dobereiner believe that lithium, sodium, and potassium belonged in a triad?
- What was a shortcoming of the triad system?
- How did Newlands arrange the element?
- What was a problem with the “Law of Octaves"?

