4.3: Law of Multiple Proportions
- Page ID
- 52750
What are the similarities and differences between a unicycle and a bicycle?
Just from the words themselves, the astute Latin-speaking scholar can tell that, whatever it is made of, the unicycle has one of them (uni = "one") and the bicycle has two (bi = "two"). From pictures, we get additional information that helps us tell the two apart. The unicycle has one wheel and the bicycle has two. In particular, they are made up of the same materials, and the only significant difference is the number of wheels on the two vehicles. Now—how many wheels are on a tricycle?
Law of Multiple Proportions
Once the idea that elements combined in definite proportions to form compounds was established, experiments also began to demonstrate that the same pairs of certain elements could combine to form more than one compound. Consider the elements carbon and oxygen. Combined in one way, they form the familiar compound carbon dioxide. In every sample of carbon dioxide, there are \(32.0 \: \text{g}\) of oxygen present for every \(12.0 \: \text{g}\) of carbon. By dividing \(32.0\) by \(12.0\), this simplifies to a mass ratio of oxygen to carbon of 2.66 to 1. There is another compound that forms from the combination of carbon and oxygen called carbon monoxide. Every sample of carbon monoxide contains \(16.0 \: \text{g}\) of oxygen for every \(12.0 \: \text{g}\) of carbon. This is a mass ratio of oxygen to carbon of 1.33 to 1. In the carbon dioxide, there is exactly twice as much oxygen present as there is in the carbon monoxide. This example illustrates the law of multiple proportions: whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.
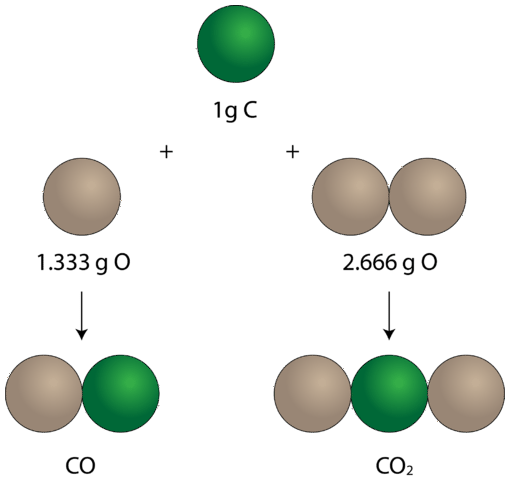
In carbon monoxide, on the left, there is \(1.333 \: \text{g}\) of oxygen for every \(1 \: \text{g}\) of carbon. In carbon dioxide, on the right, there is \(2.666 \: \text{g}\) of oxygen for every gram of carbon. So the ratio of oxygen in the two compounds is 1:2, a small whole number ratio.
The difference between carbon monoxide and carbon dioxide is significant. Carbon monoxide is a deadly gas, formed from the incomplete combustion of some carbon-containing materials (such as wood and gasoline). This compound will attach to hemoglobin in the red blood cells and block the binding of oxygen to those cells. If oxygen does not bind, it cannot be carried to the cells of the body where it is needed, and death can occur. Carbon dioxide, on the other hand, is not toxic like carbon monoxide is. However, it can displace oxygen in systems since it is heavier. Carbon dioxide fire extinguishers cut off the flow of oxygen in a fire, putting out the fire.
Summary
- The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.
Review
- State the law of multiple proportions.
- In carbon dioxide (CO2), how many grams of oxygen (O) would there be if there are 24 grams of carbon (C)?
- How many grams of carbon (C) would be present in carbon monoxide (CO) that contains 2.666 grams of oxygen (O)?

