9.15: Molecular Shapes - Lone Pair(s) on Central Atom
- Page ID
- 53755

How can all these clothes fit into such a small space?
When we travel, we often take a lot more stuff than we need. Trying to fit it all into a suitcase can be a real challenge. We may have to repack or just squeeze it all in. Atoms often have to rearrange where the electrons are in order to create a more stable structure.
Central Atom with One or More Lone Pairs
The molecular geometries of molecules change when the central atom has one or more lone pairs of electrons. The total number of electron pairs, both bonding pairs and lone pairs, leads to what is called the electron domain geometry. When one or more of the bonding pairs of electrons is replaced with a lone pair, the molecular geometry (actual shape) of the molecule is altered. In keeping with the A and B symbols established in the previous section, we will use E to represent a lone pair on the central atom (A). A subscript will be used when there is more than one lone pair. Lone pairs on the surrounding atoms (B) do not affect the geometry.
AB\(_3\)E: Ammonia, \(\ce{NH_3}\)
The ammonia molecule contains three single bonds and one lone pair on the central nitrogen atom (see figure below).
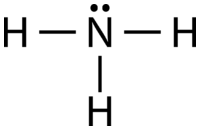
The domain geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{CH_4}\). In the ammonia molecule, one of the electron pairs is a lone pair rather than a bonding pair. The molecular geometry of \(\ce{NH_3}\) is called trigonal pyramidal (see figure below).
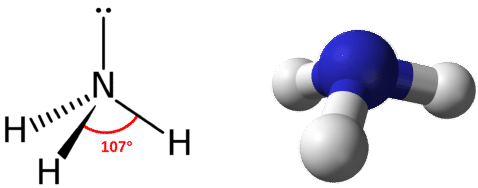
Recall that the bond angle in the tetrahedral \(\ce{CH_4}\) molecule is \(109.5^\text{o}\). Again, the replacement of one of the bonded electron pairs with a lone pair compresses the angle slightly. The \(\ce{H-N-H}\) angle is approximately \(107^\text{o}\).
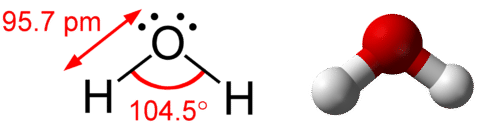
AB\(_2\)E\(_2\): Water, \(\ce{H_2O}\)
A water molecule consists of two bonding pairs and two lone pairs (see figure below).

As for methane and ammonia, the domain geometry for a molecule with four electron pairs is tetrahedral. In the water molecule, two of the electron pairs are lone pairs rather than bonding pairs. The molecular geometry of the water molecule is bent. The \(\ce{H-O-H}\) bond angle is \(104.5^\text{o}\), which is smaller than the bond angle in \(\ce{NH_3}\) (see figure below).
AB\(_4\)E: Sulfur Tetrafluoride, \(\ce{SF_4}\)
The Lewis structure for \(\ce{SF_4}\) contains four single bonds and a lone pair on the sulfur atom (see figure below).
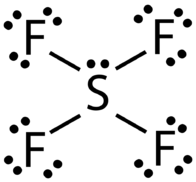
The sulfur atom has five electron groups around it, which corresponds to the trigonal bipyramidal domain geometry, as in \(\ce{PCl_5}\) (see figure below). Recall that the trigonal bipyramidal geometry has three equatorial atoms and two axial atoms attached to the central atom. Because of the greater repulsion of a lone pair, it is one of the equatorial atoms that are replaced by a lone pair. The geometry of the molecule is called a distorted tetrahedron, or seesaw.
| Total Number of Electron Pairs | Number of Bonding Pairs | Number of Lone Pairs | Electron Domain Geometry | Molecular Geometry | Examples |
|---|---|---|---|---|---|
| 3 | 2 | 1 | Trigonal Planar | Bent | \(\ce{O_3}\) |
| 4 | 3 | 1 | Tetrahedral | Trigonal Pyramidal | \(\ce{NH_3}\) |
| 4 | 2 | 2 | Tetrahedral | Bent | \(\ce{H_2O}\) |
| 5 | 4 | 1 | Trigonal Bipyramidal | Distorted Tetrahedron (Seesaw) | \(\ce{SF_4}\) |
| 5 | 3 | 2 | Trigonal Bipyramidal | T-shaped | \(\ce{ClF_3}\) |
| 5 | 2 | 3 | Trigonal Bipyramidal | Linear | \(\ce{I_3^-}\) |
| 6 | 5 | 1 | Octahedral | Square Pyramidal | \(\ce{BrF_5}\) |
| 6 | 4 | 2 | Octahedral | Square Planar | \(\ce{XeF_4}\) |
Summary
- The presence of lone pair electrons influences the three-dimensional shape of the molecule.
Review
- Why does water have a bent geometry?
- Why is ammonia not a planar molecule?
- How would we write the configuration for xenon tetrafluoride using the ABE system?

